Question
Question: Explain why Aniline with \({\text{B}}{{\text{r}}_{\text{2}}}{\text{/O}}{{\text{H}}^{\text{ - }}}\) g...
Explain why Aniline with Br2/OH - gives 2,4,6 - tribromoaniline, while phNMe2 gives meta nitro derivative when mono nitrated with HNO3/H2SO4.
Solution
Aniline is an organic compound with the formula C6H5NH2. With a phenyl group attached to an amino group, aniline is the simplest aromatic amine. Chemically, it has an electron-rich benzene derivative which reacts rapidly in electrophilic aromatic substitution reactions. Aniline is a weaker base and a poorer nucleophile than other structurally similar aliphatic amine.
Complete step by step answer:
Aniline is a benzene ring with an NH2 group attached to it. And this NH2 group has a lone pair of electrons and the hybridization is between sp3 and sp2. Because of which the lone pair is in an spx hybrid orbital with high p character. The amino group in aniline is flatter than that in an aliphatic amine, which helps in conjugation of lone pairs with the aryl substituent.
Here, a lone pair of electrons on nitrogen atom in NH2 group spread into the benzene ring and delocalization of electron occur. The negative charge develops on the ortho and para (O AND P) positions and the change in density is higher during delocalization.
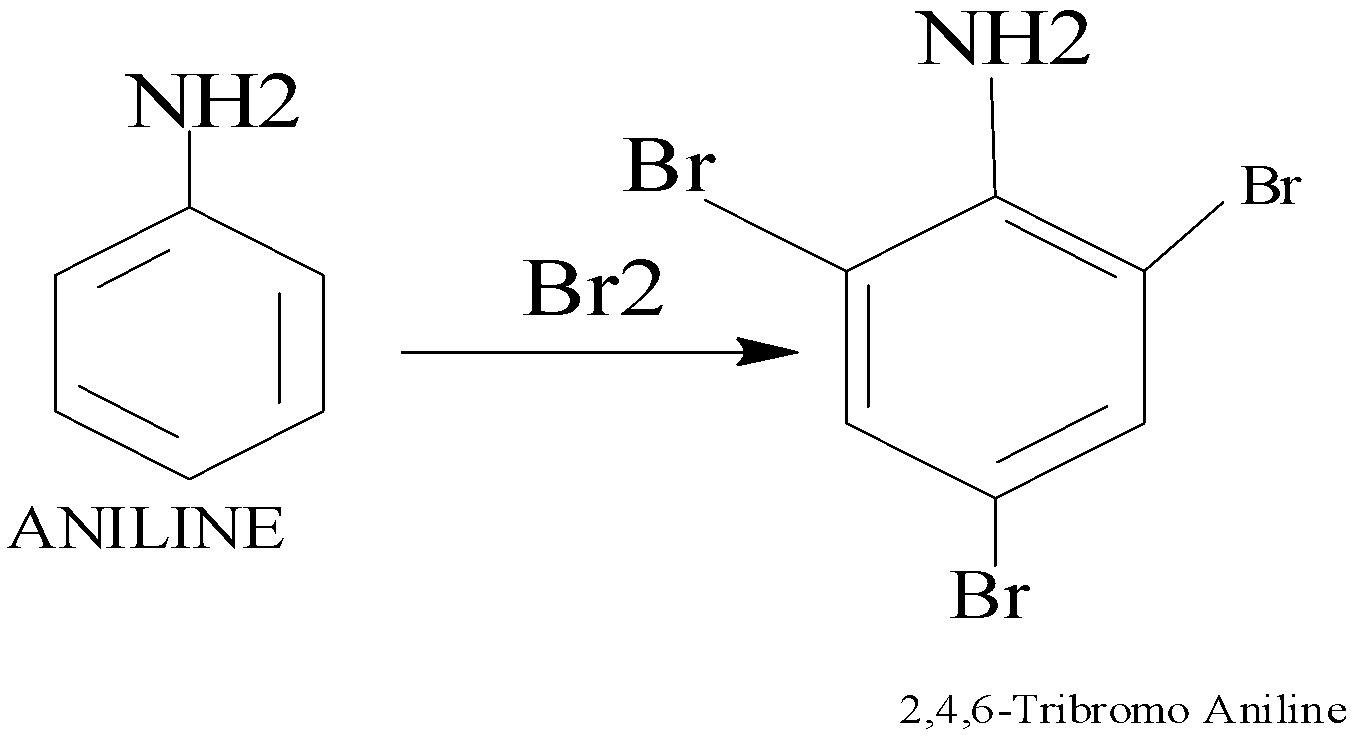
So, - NH2 group, activates the ortho and para positive.
When bromine water is added to aniline, the bromine water is decolourised and a white precipitate of 2,4,6, tribromo aniline is formed.
To generate the mono-substituted product i.e., 4-bromo aniline, a protection with acetyl chloride is provided and then hydrolysed back to reform aniline.
PhNMe2 acts as a base in acidic condition and the formation of NHMe2 which is meta directing group when mono nitrated with HNO3/H2SO4.
Additional Information:
Aniline is highly susceptible to electrophilic reactions which shows that it is an enamine which enhances the electron donating ability of the ring. For example, reaction of aniline with sulfuric acid (H2SO4) at 180oc produces sulfanilic acid (H2NC6H4SO3H).
Note: The pyramidal geometry is because of two factor 1) stabilization of the N lone pair in an orbital with scharacter which favors pyramidalization 2) delocalization of the Nlone pair into the aryl ring favors planarity (giving the best overlap between the orbitals of the benzene ring and a lone pair in a pure p orbital)
