Question
Question: Explain whether the compound shown below is aromatic or not? 
Solution
Hint : For a compound to be aromatic it must follow some rules which are as follows:
-The compound or molecule must be cyclic and planar i.e., each carbon must be sp2 hybridized.
-The molecule must have conjugated pi bonds.
-The molecule must follow Huckel’s rule i.e., it must consist of a (4n+2)π electron system.
Complete Step By Step Answer:
Quasi aromatic compounds: These are the type of aromatic compounds in which charge is present on the ring which participate in the conjugation to show aromatic behaviour. In simple words, a quasi-aromatic compound is ionic in nature with a counter ion which takes part in the (4n+2)π electron system of the compound.
As per question, the given compound is as follows:
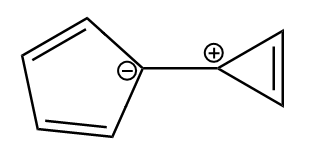
As the compound consists of two ions, let’s look at the aromaticity of each ion separately.
Given anion is as follows:
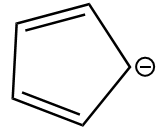
The ion is cyclic and each carbon atom is sp2 hybridized i.e., it is planar as well. The value of n according to the Huckle rule for the given anion is as follows:
4n+2=6
⇒4n=4
⇒n=1
The negative charge on the ion participate in conjugation as follows:
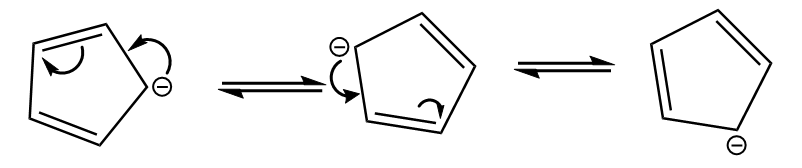
As it satisfies all the conditions for aromaticity, hence it is an aromatic compound.
Given cation is as follows:
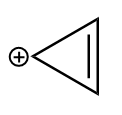
The ion is cyclic and each carbon atom is sp2 hybridized i.e., it is planar as well. The value of n according to the Huckle rule for the given anion is as follows:
4n+2=2
⇒4n=0
⇒n=0
The positive charge on the ion participate in conjugation as follows:
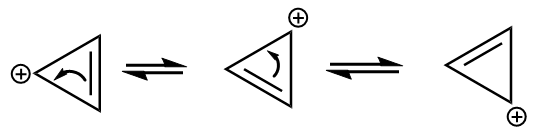
As it satisfies all the conditions for aromaticity, hence it is an aromatic compound.
Therefore, we can conclude that the given molecule is aromatic because it is composed of two aromatic molecules and because of the presence of charge, it is categorized as a quasi-aromatic compound.
Note :
It is important to note that for a compound to be aromatic, the value of n used in the Huckle rule must be a non-negative integer i.e., n=1,2,3,4⋅⋅⋅ . If a compound obeys a 4nπ electron system, then it is said to be an antiaromatic compound.
