Question
Question: Explain the Wurtz-Fittig Reaction....
Explain the Wurtz-Fittig Reaction.
Solution
Wurtz-Fittig reaction is the reaction in which alkyl and aryl halides react in the presence of dry ether and sodium metal to give alkyl-aryl substituted product. Alkyl halide is composed of aliphatic hydrocarbons and aryl halides are composed of aromatic hydrocarbons like phenyl or naphthyl group.
Complete Solution :
This reaction was investigated by Charles Adolphe Wurtz and Wilhelm Rudolph Fittig, therefore, it is named as Wurtz-Fittig Reaction. This reaction can be explained on the basis of radical mechanisms.
- This is a type of coupling reaction in which there is formation of carbon-carbon bond by the reaction between aryl halide and alkyl halide during the presence of sodium metal and dry ether. It could be used in the formation of higher alkanes. The basic schematic reaction is given below-
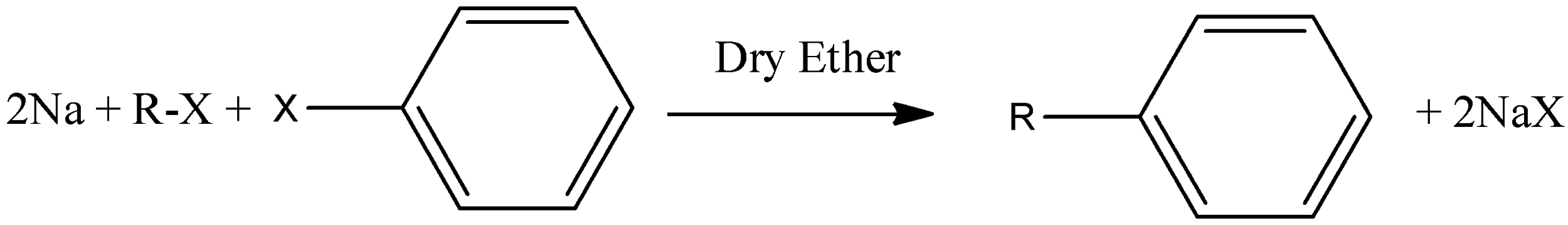
The mechanism is mentioned below-
At first, Sodium radical is formed in the presence of dry ether and then reacts with alkyl halides to form the alkyl radical.
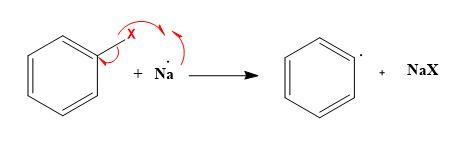
- Similarly, sodium metal will also react with aryl halides to form aryl radicals.

- At last, we can see the alkyl and aryl radicals that formed in the above two steps will lead to the formation of substituted aromatic compound as shown below-
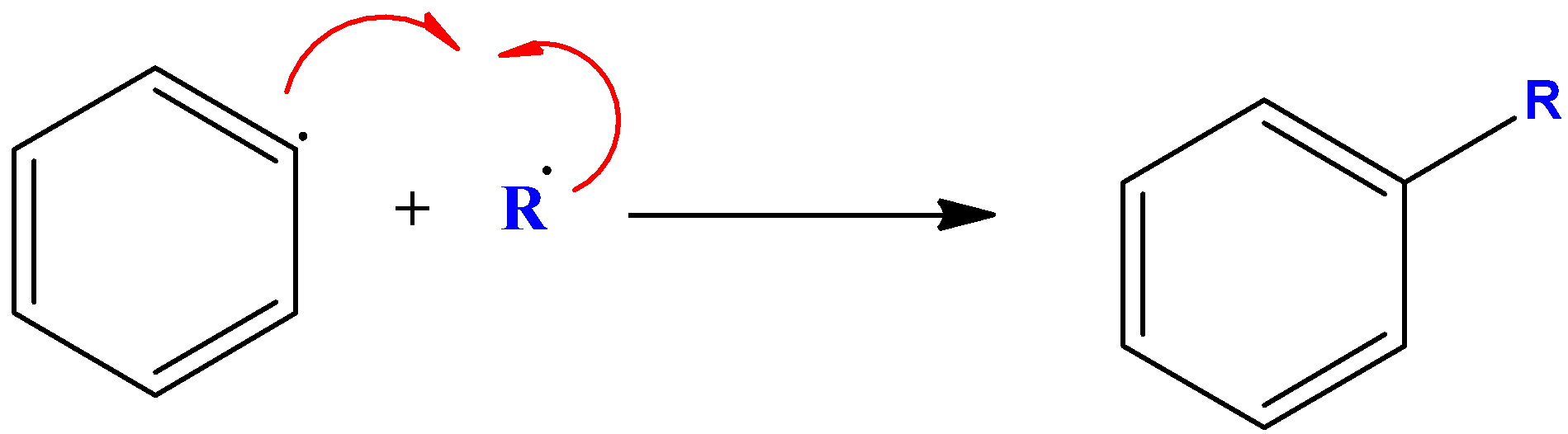
One thing that should be noted is that the reaction requires aprotic solvent as the medium if reaction. That is why dry ether was chosen as the suitable solvent because it is a non-polar aprotic solvent.
Note: The most important part of the reaction that should be noted is the difference among Wurtz, Fittig and Wurtz-Fittig reaction.
(i) In Wurtz Reaction, two aliphatic alkyl halides react in presence of dry ether and sodium metal to set up higher alkanes.
2R−X+2NaDry etherR−R+2NaX
(ii) In Fittig reaction, two aryl (aromatic rings) halides react in presence of dry ether and sodium metal to form biphenyls.
R−X+Ph−X+2NaDry EtherR−Ph+2NaX
