Question
Question: Explain the violet color of \[{\left[ {{\mathbf{Ti}}{{\left( {{{\mathbf{H}}_{\mathbf{2}}}{\mathbf{O}...
Explain the violet color of [Ti(H2O)6]3+complex on the basis of the Crystal Field theory?
Solution
In the given question we will discuss how the change of the color of the complex occurs on the basis of the crystal field theory. The CFT is a theory which is used to describe many spectroscopies of transition metal coordination complexes, in specific optical spectra that are colors.
Complete answer:
In the Coordination compounds they exhibit color, which is credited to the crystal field theory, that corresponds to the d−d transition of the elements. In ground state, the complex of Titanium has 23 electrons which have the outer shell electronic configuration that is 3d34s2 and in the complex it is observed that titanium is in its +3 oxidation state. This is accomplished by losing 3 electros. So, it has the configuration 3d2. Since it has 2 unpaired electrons and has the ability to undergo d−d transition, thus the complex is colored.
When light corresponding to the energy of the yellow-green region is absorbed by the complex, this would excite the electron fromt2g level to the eg level. Thus, the complex occurs in violet color.
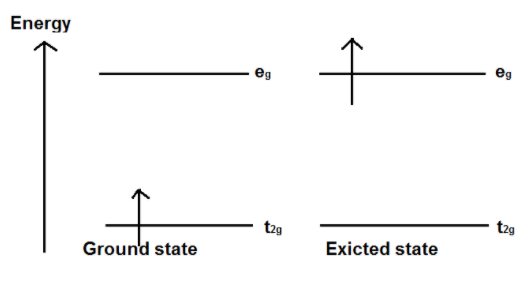
Figure 1 transition of electron in Ti complex
As though in the absence of ligands there will be no crystal field splitting and substance become colorless. when water molecules are removed it becomes colorless.
Note: When the excitation of an electron takes place from the ground state to excited state, electrons will absorb light that will excite the electron from one energy level to another and will return to ground state in the form of colors.
