Question
Question: Explain the structure of diborane ....
Explain the structure of diborane .
Solution
Diborane is colourless having chemical formula of B2H6.it is toxic in case inhaled. It has an offensive odor. Diborane is formed by boric acid and water. It is easily mixed with air and becomes explosive . so, it must be handled carefully .
Complete answer:
Diborane is made up of a total eight hydrogen atoms and two borons . In which two hydrogen are on the same plane. While the other two hydrogen forms a bridge. Bridge hydrogens are above and below the plane. These bonds are also called banana bonds . The hybridisation of Diborane is sp3 in which the length of B−Hbridge is 1.33 and length of B−Hterminal is 1.19 A∘.
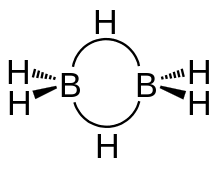
in the diborane the two boron do not form any bonds . The angle between the two terminal hydrogen and one boron is 120∘ and the angle between the bridge hydrogen and
boron is 97∘
Additional information:
Borane can be formed by the reaction of sodium tetrahydridoborate and boron trifluoride etherate . This method is considered the most convenient process for manufacturing diborane .
it can't exist as BH3 because boron has three electrons in its valence shell . It is an electron deficient compound and unstable . As after forming a bond with three hydrogens the total number of electrons around the boron is six so it is considered as electron deficient and thus it exists as a dimer which we call diborane .
Note:
Diborane is used in rubber vulcaniser , rocket propellants and a doping agent in the manufacture of semiconductors.It is also used as a reducing agent. It releases a huge amount of energy when burnt in oxygen.
