Question
Question: Explain the structure of diamond with a figure?...
Explain the structure of diamond with a figure?
Solution
We have to know that diamond is a strong type of the component carbon with its particles masterminded in a precious stone construction called precious stone cubic. At room temperature and pressing factor, another strong type of carbon known as graphite is the synthetically steady type of carbon, however diamond never converts to it.
Complete answer:
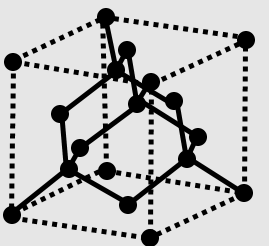
We have to know that diamond has the most noteworthy hardness and warm conductivity of any normal material, properties that are used in major modern applications like cutting and cleaning apparatuses. They are likewise the explanation that diamond blacksmith's iron cells can expose materials to pressures discovered somewhere down in the Earth. Since the course of action of particles in diamond is incredibly unbending, barely any sorts of pollution can taint it (two exemptions being boron and nitrogen). Little quantities of deformities or pollution (around one for each million of grid atoms) shading jewel blue (boron), yellow (nitrogen), earthy colored (deserts), green (radiation openness), purple, pink, orange, or red. Diamond additionally has generally high optical scattering (capacity to scatter light of various tones).
When synthetic diamond can be developed from high-virtue carbon under high pressing factors and temperatures or from hydrocarbon gas by synthetic fume testimony. Impersonation precious stones can likewise, be made out of materials like cubic zirconia and silicon carbide. Characteristic, synthetic and impersonation diamonds are most regularly recognized utilizing optical strategies or warm conductivity estimations.
Note:
We have to know that diamond is a strong type of unadulterated carbon with its atoms masterminded in a gem. Strong carbon comes in various structures known as allotropes relying upon the sort of substance bond. The two most regular allotropes of unadulterated carbon are precious stone and graphite.
