Question
Question: Explain the \(s{p^2}\) hybridisation?...
Explain the sp2 hybridisation?
Solution
As we know that the atomic orbitals combine to form a new set of equivalent orbitals known as the hybrid orbitals and it helps in explaining the shape of molecules and equivalency of bonds. It can be classified into sp,sp2,sp3 and so on which involves one s-orbital and one p-orbital, one s and two p-orbitals etc.
Complete answer:
As we know that hybridisation is a theoretical concept which has been introduced to explain the structural properties such as shape of molecule and equivalency of bonds etc which cannot be explained by simple theories of valency.
We also know that there are many different types of hybridisation depending upon the types of orbitals involved in mixing like sp,sp2,sp3,sp3d etc. let us talk about sp2 hybridisation among these.
We can see that in sp2 hybridisation, one s-orbital and two p- (px&py) orbitals of one atom hybridise to give three equivalent sp2 hybrid orbitals. These three sp2 hybrid orbitals are directed towards the three corners of an equilateral triangle with an angle of 120∘ and give a triangular geometry to the molecule. We can represent it as:
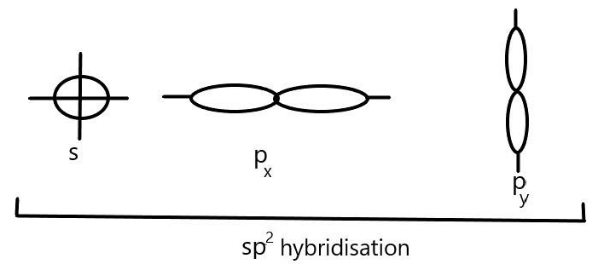
sp2 hybrid orbitals are larger in size than the sp-hybrid orbitals but slightly smaller than that of sp3. Examples of sp2 hybridisation includes the compounds of boron like BF3,BCl3 and BH3 as well as aluminium and carbon containing compounds such as AlCl3 and CH2=CH2respectively.
Note: Always remember that sp2 hybridisation involves the mixing of one s-orbital and two p-orbitals which includes the promotion of one electron in the s-orbital and the one to the any one of the p-orbital whose combination creates a three new hybrid orbitals of equivalent energy levels. The sp2 hybridised orbitals possess a trigonal planar geometry of molecules and these hybrid orbitals are more effective in forming stable bonds than the pure atomic orbitals.
