Question
Question: Explain the Reimer-tiemann reaction with an example:...
Explain the Reimer-tiemann reaction with an example:
Solution
The reimer tiemann reaction is a type of reaction which is used to convert phenol to salicylic acid with the use of chloroform.
In the reaction, the electrophilic substitution occurs and it could be understood with the use of the reaction mechanism.
Complete step-by-step answer:
In the reaction, the phenol is treated with chloroform. So, at first we will be discussing the structure of both for a better understanding of the mechanism. In the chemical reaction, the structures of the chemicals are the most important and basic part of the mechanisms. Now if we consider the structure of phenol, it contains a benzene ring and an alcoholic group is attached to that ring. During resonance, this alcoholic group participates in resonance too, as it has electrons in the oxygen group, it activates the ring for electrophilic substitution at ortho and para positions.
Now if we consider the chloroform, we know that it has the formula, CHCl3, which can act as an electrophile if one of those chlorine atoms are removed. Now, since some of the positions in the phenol ring are active for electrophilic substitution, it will get attached to the most appropriate position, which will result in most stability.
For a better understanding, we will be considering the reaction mechanism.
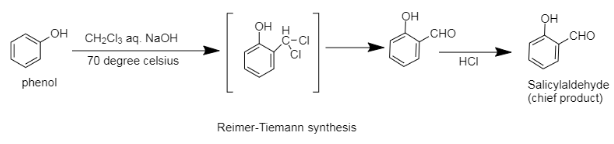
Now as we can see that, upon reaction of phenol with chloroform, an intermediate is formed and then after removal of two chlorines the carbon of the electrophile acquires an oxygen atom from the solution mixture, which ultimately results in the formation of salicylaldehyde.
The electrophile chooses the ortho position instead of availability of the para position because the para position is far from the oxygen of the phenoxide ion and so it experiences lesser inductive effect and has lesser electron density than ortho position.
Note: In the reaction of Riemer-Tiemann, the phenol is first converted to phenoxide ion which starts the whole mechanism of the reaction.
But as we know that the inductive effect tends to decrease with increase in distance, so the ortho position, being closer to the oxygen, becomes a more appropriate position for the electrophile to get attached to.
