Question
Question: Explain the molecular orbital structure of benzene....
Explain the molecular orbital structure of benzene.
Solution
: The benzene molecule contains six carbon atoms and each carbon is sp2hybridized, and the 2pz orbitals overlap to give two sets of πbonds.
Complete step-by-step answer:
Benzene is an organic compound that has the formula of C6H6. It is an aromatic compound and consists of a single ring structure . The carbon atoms are sp2 hybridised in benzene and all of them lie in the same plane. They have a 1200angle orientation. Perpendicular to the plane of the hybrid orbital, there exists an unhybridized p orbital which contains two lobes. There are three sp2hybrid orbitals of each carbon and from these, two of the orbitals overlap interracially, with neighbouring orbitals so that σ−σcarbon bonds are formed.
The leftover third hybrid orbital of each carbon undergoes overlapping with the hydrogen’s half filled 1s orbital and forms σC−σH bond. So, in total there are 12 sigma bonds, where 6 of them are carbon-carbon and the other six are carbon-hydrogen. However, in each carbon atom there exists one unhybridized 2pz orbital.
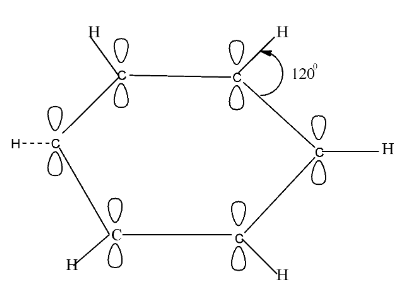
The 2pz orbital which is unhybridized overlaps with the adjacent carbon atom’s 2pz orbital and forms a continuous πmolecular network, and incorporates the six π electrons.
Finally, as a result of this, we get the formation of two clouds of electrons shaped in form of rings, one above and one below the plane of atoms.
Note: It is to be noted that the electrons in the benzene ring are delocalised, and this delocalisation provides stability to the benzene molecule. The bond length of carbon attacked to another carbon is 139 pm.
