Question
Question: Explain the mechanism of the following reaction: \({\text{2C}}{{\text{H}}_3} - {\text{C}}{{\text{H...
Explain the mechanism of the following reaction:
2CH3−CH2−OHH+413 KCH3−CH2−O−CH2−CH3+H2O
Solution
In the reaction, the reactant has a hydroxyl (−OH) functional group and the product has ether (R−O−R) functional group, where R is any alkyl group. Thus, the reaction is conversion of alcohol to ether.
Complete step by step answer:
The given reaction is,
2CH3−CH2−OHH+413 KCH3−CH2−O−CH2−CH3+H2O
In the given reaction, excess ethyl alcohol reacts with hydrogen ion from the sulphuric acid. In the reaction, diethyl ether is produced along with a water molecule.
The reaction mechanism involves three steps as follows:
Step 1: Formation of protonated alcohol:
In this step, one molecule of ethyl alcohol undergoes protonation i.e. a proton or hydrogen ion gets attached to the oxygen atom of the hydroxyl functional group. It is a fast step.
The reaction is as follows:
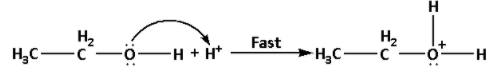
Step 2: Nucleophilic attack of the alcohol molecule on the protonated alcohol:
In this step, the second molecule of ethyl alcohol attacks the protonated alcohol molecule formed in the first step. It is a slow step.
The reaction is as follows:
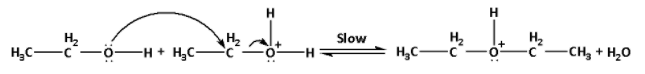
Step 3: Deprotonation to form ether:
In this step, the intermediate formed undergoes deprotonation i.e. a proton or a hydrogen ion gets removed. It is a fast step.
The reaction is as follows:
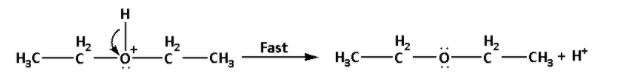
Note: In the reaction, equal volumes of ethyl alcohol and concentrated sulphuric acid are distilled. Ethyl alcohol reacts with concentrated sulphuric acid and produces ethyl hydrogen sulphate. The reaction is as follows:
CH3−CH2−OH+H−O−SO3H→CH3−CH2−O−SO3H+H2O
Then excess of ethyl alcohol is added. Ethyl hydrogen sulphate reacts with excess of ethyl alcohol and produces diethyl ether. The reaction is as follows:
CH3−CH2−OH+CH3−CH2−O−SO3H→CH3−CH2−O−CH2−CH3+H2SO4
The sulphuric acid is regenerated in the reaction. This process is known as a continuous esterification process.
