Question
Question: Explain the mechanism of alkaline hydrolysis of t-butyl bromide with energy profile diagram....
Explain the mechanism of alkaline hydrolysis of t-butyl bromide with energy profile diagram.
Solution
The alkaline hydrolysis of t-butyl bromide is a first order reaction. This is because of the fact that the rate of the reaction entirely depends on t-butyl bromide. The energy profile diagram is a representation of the energy path followed by the reactants to form products.
Complete answer:
Let us write the equation for alkaline hydrolysis of t-butyl bromide with aqueous alkali such as NaOH or KOH.
(CH3)3C−Br+NaOHaq(CH3)3−OH+NaBr
In this reaction, we can see that the Bromine atom is substituted by OH. Hence this is a nucleophilic substitution reaction. The rate of this reaction only depends only on t-butyl bromide and hence this is a first order reaction.
This reaction follows the SN1 mechanism and hence it involves a two step mechanism.
Step 1: This first step consists of the breakage of C−Br bond. This step is the slow step or the rate determining step. The rate of the reaction depends on this step.
We can write the equation for this reaction as follows.
(CH3)3C−Brslow(CH3)3C++Br−
This reaction involves the formation of an intermediate which is an unstable carbocation.
Step 2: This step consists of bond formation between intermediate carbocation and the OH anion. This step is the fast step. The equation for this step can be written as follows.
(CH3)3C++OH−fast(CH3)3C−OH
The energy profile diagram can be drawn as:
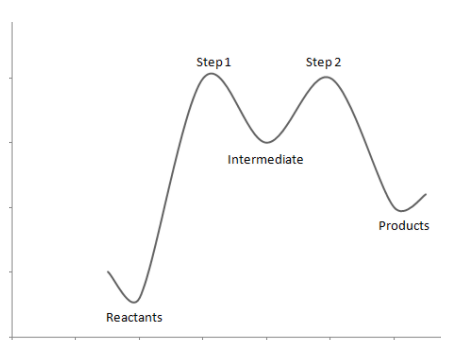
Note: It is to be noted that in the energy profile diagram, the reactants i.e. t-butyl bromide has the least energy. Through step one and step two reactions, the compound gains energy as the intermediates are unstable. Once the products are formed the energy decreases indicating that the product formed is stable.
