Question
Question: Explain the industrial production of phenol of high purity and less production cost and also explain...
Explain the industrial production of phenol of high purity and less production cost and also explain the bromination of phenol.
Solution
We know that when bromine water is added to a solution of phenol in water, the bromine water is decolorized and a white precipitate is formed which smells of antiseptic. We know that the reaction of phenol and water with bromine is known as bromination of phenol. Solvent has a great influence on reaction.
Complete answer:
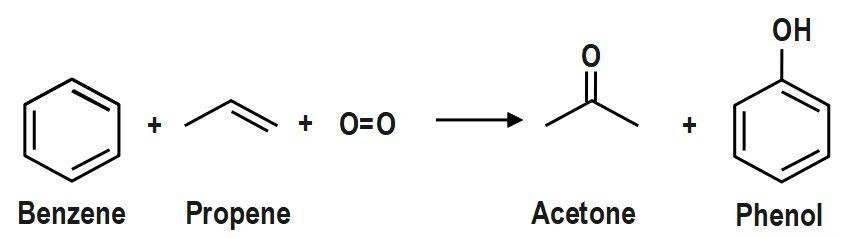
The cumene method (cumene-phenol method, Hock process) is an associate process for synthesizing phenol and dimethyl ketone from benzene and gas. This method converts two comparatively low-cost beginning materials, benzine and gas, into a lot of valuable ones, phenol and dimethyl ketone. Different reactants needed are atomic numbers from air and tiny amounts of a radical instigator.
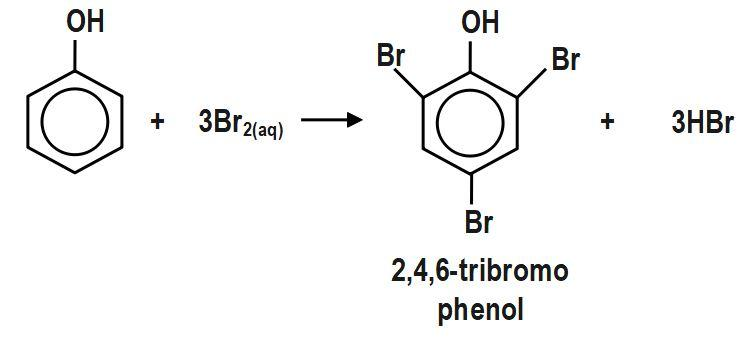
Reaction of phenol with halogen is understood as bromination of phenol. Solvent has a nice influence on the reaction. In different solvents, different merchandise are obtained. Action of halogen on phenol is explained as reaction with halogen in water: Phenol reacts with halogen water to provide 2,4,6−tribromophenol. In water ionization is facilitated. Phenol gets ionized to create phenoxide particles, which is an even higher ortho-para directive. Bromine additionally gets ionized to a larger extent to create a sizable amount of bromonium ions.
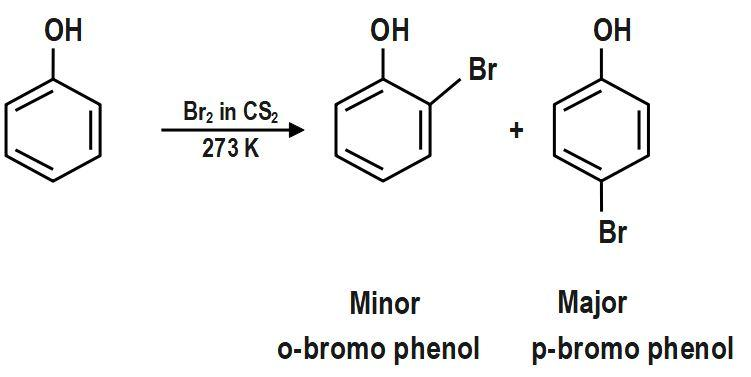
Reaction with halogen in CS2: Phenol reacts with halogen in presence of Carbon disulphide to create a mixture of o-bromophenol and p-bromophenol. Among that p-bromophenol predominates. In CS2 ionization isn't facilitated that a lot of.
Note:
Remember that Bromination of phenol is a substitution reaction. Where the bromine replaces hydrogen present in the benzene ring of phenol. The water solvent when phenol treated with gives a polybromo derivative in which all hydrogen atoms at ortho, meta, and para positions with respect to the −OH group are replaced by bromine atoms.
