Question
Question: Explain the industrial method of preparation of bleaching powder with a neat diagram....
Explain the industrial method of preparation of bleaching powder with a neat diagram.
Solution
Bleaching powder is extensively used as a disinfectant and in bleaching of paper. Bleaching powder is known by calcium hypochlorite as its chemical name. The chemical formula of bleaching powder is CaOCl2.
Complete step by step solution:
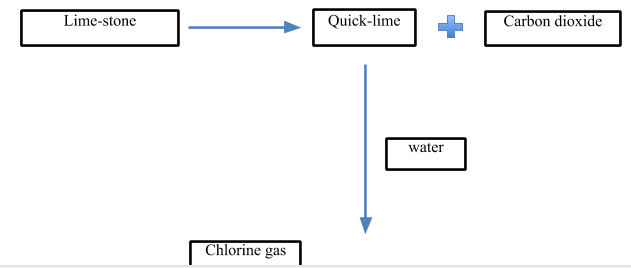
Let’s look at the process of production of bleaching powder:
- The raw material required in its production are:
- Lime-stone
- Cl2gas
Now, let’s look at the process:
- In the first step, quick lime is produced. It is produced from limestone. The limestone is heated at a very high temperature at about 898∘C to decompose it into quicklime and carbon dioxide gas.
CaCO3898∘CCaO+CO2
- In the next step, the quick lime is treated with water to give slaked lime which is the major raw material for the production of bleaching powder.
CaO+H2O→Ca(OH)2
- In the last step, the slaked lime is treated with Cl2 gas to give bleaching powder.
- The chlorine gas is passed through the slaked lime in chlorine chambers where solid bleaching powder is collected as the product. The temperature is kept below 35∘C.
- The slaked lime is poured from above while the chlorine gas is passed from a lower level.
Ca(OH)2+Cl2→Ca(ClO)2CaCl2Ca(OH)2.2H2O
Note: Students can get confused in the chemical formula of bleaching powder. CaOCl2is the representation of bleaching powder while the actual formula is Ca(ClO)2CaCl2Ca(OH)2.2H2O. So, if the formula is asked then students must choose the second one.
The bleaching action is due to hypochlorite ion and it is permanent.
