Question
Question: Explain the hybridization of the central atom in \(\text{Xe}{{\text{F}}_{\text{2}}}\text{ }\)....
Explain the hybridization of the central atom in XeF2 .
Solution
InXeF2 , the Xe atom is at the central atom. The two fluorine atoms are surrounding the Xe atom. Xe has 8 valence electrons. Two of these electrons are utilized in forming Xe−F bonds. Therefore, the total bond pairs are 2 and lone pairs are 3. Thus, XeF2 has the 5 hybrid orbitals. These 5 hybrid orbitals arranged themselves in the space to minimize the repulsion between the bond pair and lone pairs to give a definite geometry to the molecule.
Complete step by step answer:
XeF2 is the molecular formula for the compound Xenon difluoride which is a powerful fluorinating and oxidizing agent. XeF4 or xenon tetrafluoride and XeF6 or xenon hexafluoride are compounds other than XeF2 , out of which XeF2 is the most stable compound.
XeF2 appears as a white crystalline solid and is used for fluorinating purposes in electrochemical procedures and laboratories. It also has a typical nauseating odor and is easily decomposed when it comes in contact with vapor or light.
The electronic configuration of Xe is as follows:
Xe = [Kr] 4d10 5s2 5p6
The valence shell contains the 8 electrons which are accommodated on the atom.
The Lewis structure of XeF2 is given as follows.
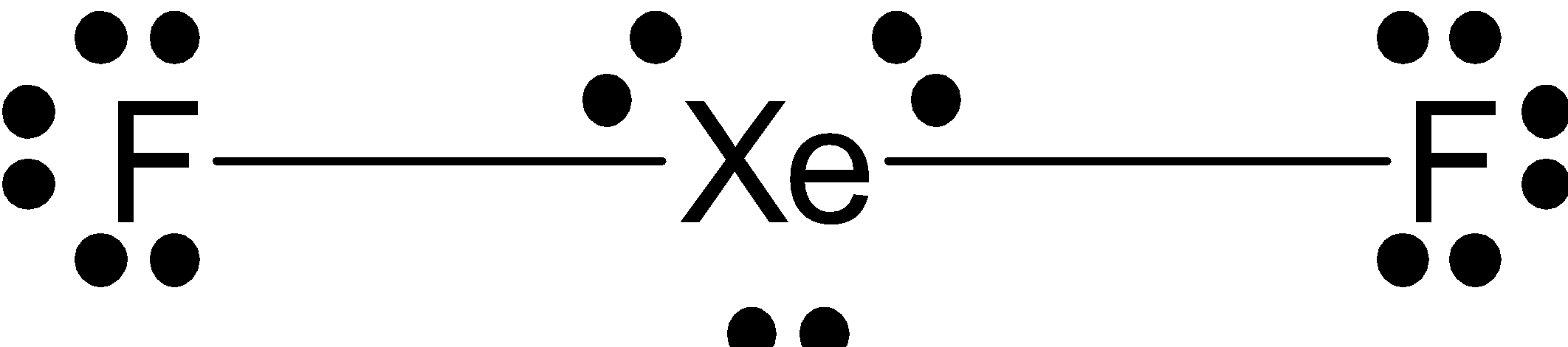
Lewis structure is based on the octet rule which states that every molecule must have 8 electrons in its outer shell of an atom to attain stability and if there are more electrons than that the compound must donate that electron, whereas if there are less electrons, the compound must accept electrons from other molecules to attain stability.
In case ofXeF2 ,
