Question
Question: Explain the formation of the \(B{{F}_{3}}\) molecule using hybridisation....
Explain the formation of the BF3 molecule using hybridisation.
Solution
We know that hybridization is the mixing of atomic orbitals into new hybrid orbitals. The many types of hybridisation includes sp3, sp2 and sp.
Complete answer:
BF3 molecule has a geometrical shape of “Trigonal Planar”. The bond angle made was 120∘ with Boron at the centre and Fluorine at the 3 vertices forming an equilateral triangle. In case of BF3, the central atom does not have an octet as it has 6 particles. It becomes an octet after addition of more bonds. The structure can be drawn as:
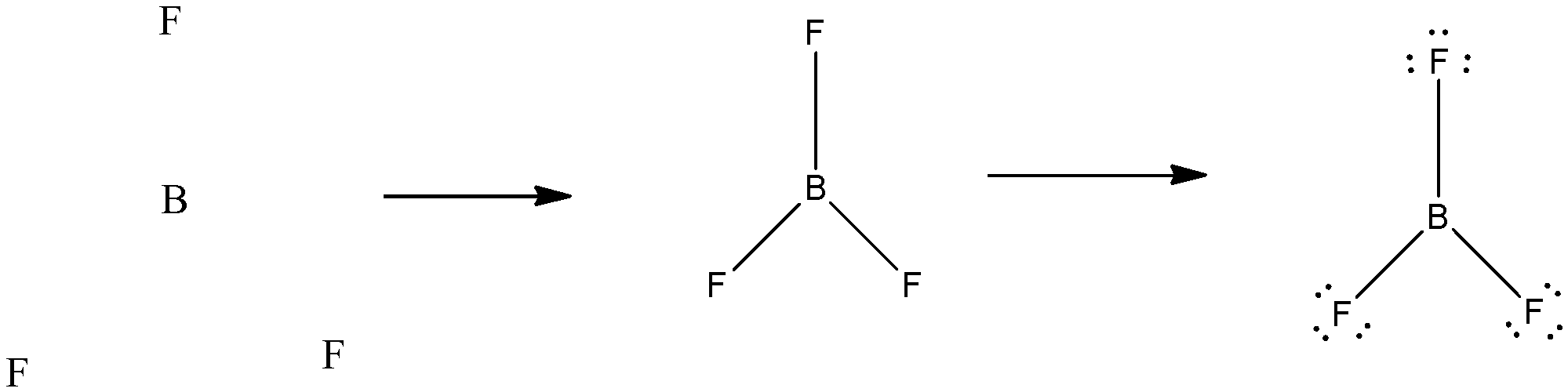
Since, a pi ( π ) bond is required for double bond between boron and 3 sigma ( !!σ!! ) bonds are formed per atom of boron, BF3is sp2 hybridised. The atomic S-orbitals and P-orbitals in the outer shell of Boron mix to form three equivalent sp2 hybrid orbitals.
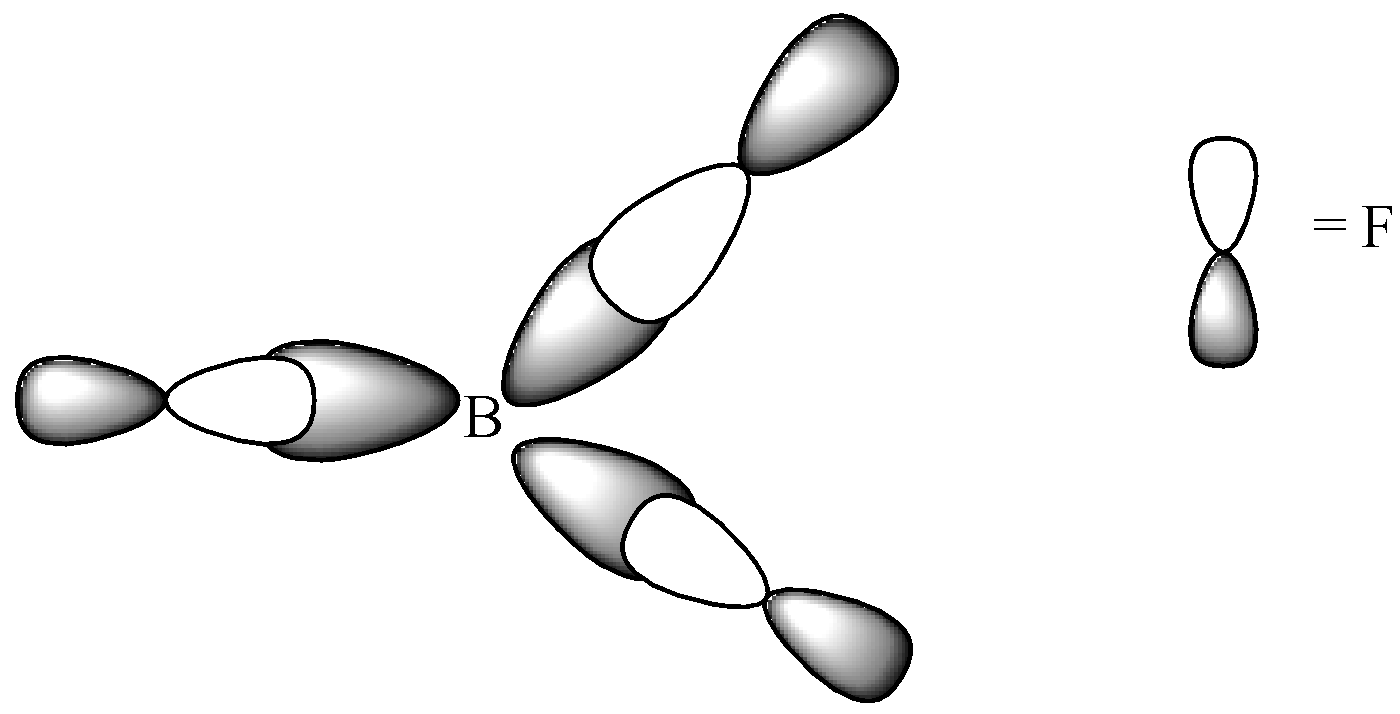
We can see that Boron has an electronic configuration 1s22s22px1 . When it is excited, the electronic configuration changes into 1s2 2s1 2px1 2py1 . Three identical B-F bonds are created in BF3 and the excited boron atom undergoes hybridisation. The intermixing and redistribution of 2s, 2px and 2py orbitals into three identical orbits form sp2 hybrid orbitals. For minimum repulsion to be present, the angle between two orbitals in the molecule must be 120∘. Each sp2 orbital must receive an electron and then 3 fluorine atoms overlap their 2pz orbitals containing unpaired electrons. Finally, the three sp2 orbitals of boron atoms contained unpaired electrons and formed three sigma-sp2 -p bonds.
Note:
We should know that boron trifluoride is electron poor and has an empty orbital. This enables it to accept a pair of electrons. Thus we can say that it is a Lewis Acid.
