Question
Question: Explain the following with one example: (a) Williamson’s synthesis (b) Kolbe’s reaction (c) He...
Explain the following with one example:
(a) Williamson’s synthesis
(b) Kolbe’s reaction
(c) Hell-volhard-zelinsky (HVZ) reaction.
(d) Aldol reaction.
Solution
In organic reactions covalent bonds break and some new covalent bonds are formed . This making and breaking of covalent bonds usually occur in a number of steps before they are finally turned into products ; the sequential description of all the steps of the transformation into product is called the mechanism of a reaction.
Complete step by step answer:
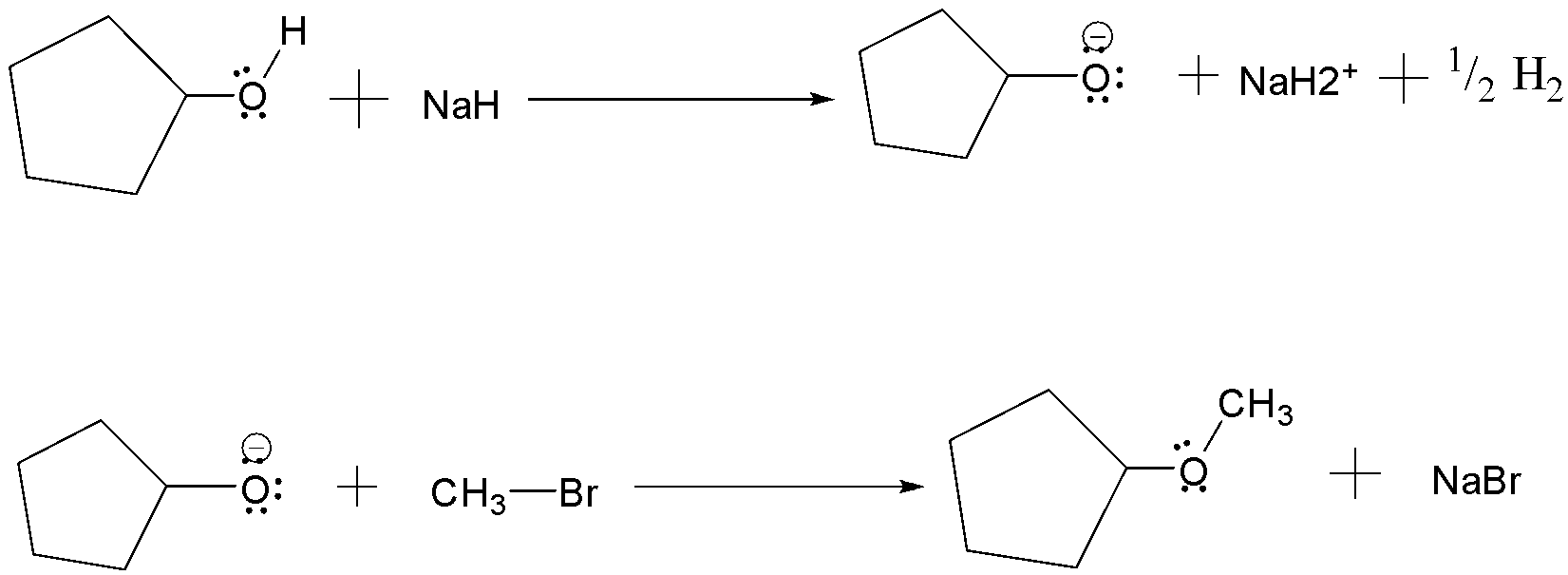
(a) Williamson’s synthesis: It is the method used for the production of ethers . It occurs by an SN2 reaction in which a metal alkoxide displaces a halide ion from an alkyl halide. The alkoxide ion is prepared by the reaction of an alcohol with a strong base such as sodium Hydride
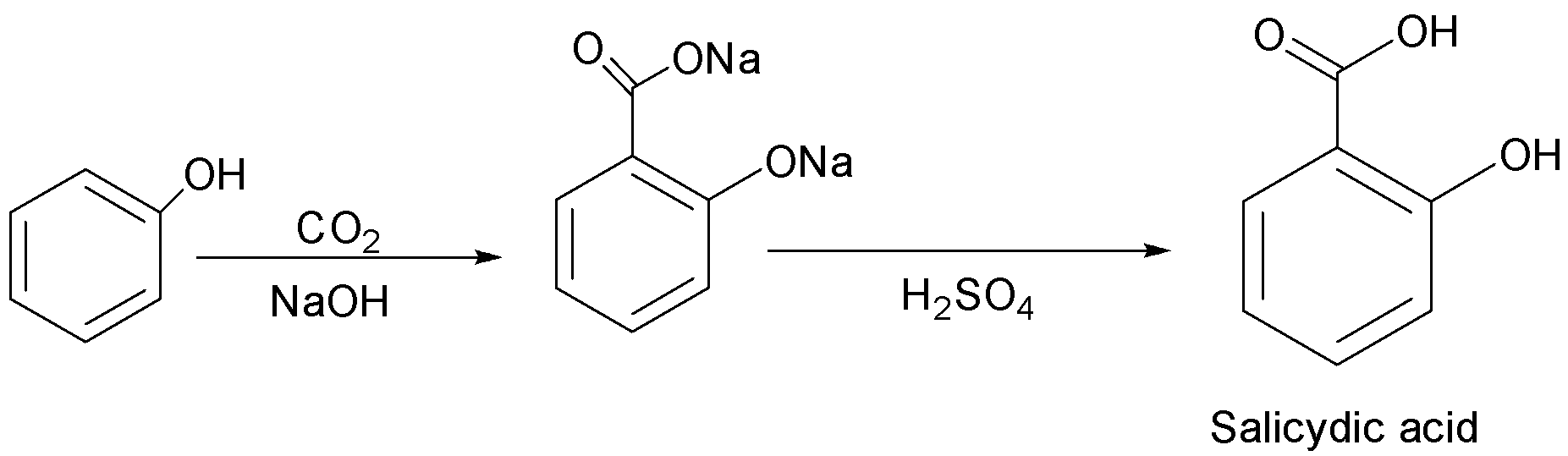
(b) Kolbe’s reaction: It is a chemical reaction that begins by first heating sodium phenoxide(the sodium salt of phenol) with carbon dioxide under pressure (100atm,125∘C) , then the product is treated with sulphuric acid. The final product is an aromatic hydroxy acid which is also known as salicylic acid.
(c) Hell-volhard-zelinsky (HVZ) reaction: When carboxylic acid reacts with chlorine or bromine using red phosphorus then halo atom is substituted at the Alpha carbon and the product obtained is mono α -halogenated acids. This is known as the HVZ reaction.
CH3CH2COOH+Br2RedPCH3CHBrCOOH+HBr
(d) Aldol reaction: The word aldol means product having both aldehyde and alcoholic group
Aldehyde having α -hydrogen(s) undergoes self condensation on warning with dilute or mild base to give β− hydroxy aldehyde, called aldol (aldehyde + alcohol). This reaction is known as aldol condensation.
Note: The mechanism of a reaction is a very important part because initially we think the reactants will produce something but when we look at the mechanism we find out that actually the product is something else .
