Question
Question: Explain the following with one example. (A) Aldol condensation (B) Cannizzaro reaction (C) Est...
Explain the following with one example.
(A) Aldol condensation
(B) Cannizzaro reaction
(C) Esterification
(D) Decarboxylation
Solution
Aldol condensation occurs between two aldehydes or a ketone molecule to form β− hydroxy aldehyde or ketone.
In Cannizzaro reaction two aldehyde molecules react to give alcohol and carboxylic acid.
The process of obtaining esters by the reaction of alcohol and carboxylic acid is known as esterification.
Decarboxylation reaction as the name suggests is the removal of CO2 by eliminating a carboxyl group.
Complete step by step answer:
In aldol condensation, the reaction of an enolate ion with a carbonyl compound in the presence of a dilute base to give β− hydroxy aldehyde or ketone. The reaction takes place only if the aldehyde has an α−H .
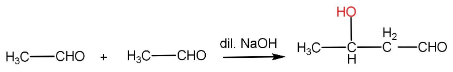
Step-I: Formation of enolate ion from 1st aldehyde molecule
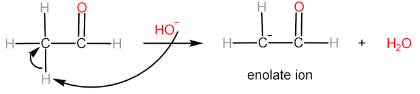
Step-II: Attack of enolate ion on 2nd aldehyde molecule
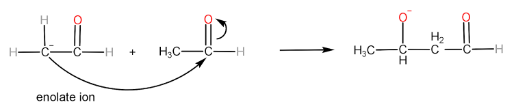
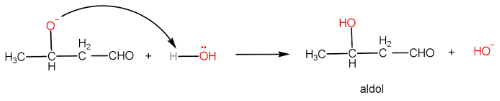
Step-III: Formation of aldol and regeneration of OH− ion
Cannizzaro reaction is the formation of a primary alcohol and a carboxylic acid molecule by the reaction of two aldehyde molecules in the presence of a strong base. The participating aldehyde molecules should not have any α−H , as such molecules readily form enolate ions and the reaction does not occur.

Step-I: Attack of OH− ion on 1st aldehyde molecule
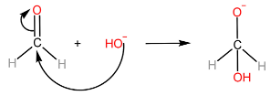
Step-II: Transfer of hydride ion to the 2nd aldehyde molecule, followed by protonation of alkoxide ion to form alcohol.
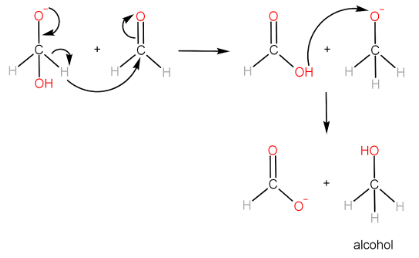
Step-III: Protonation of carboxylate ion to form carboxylic acid
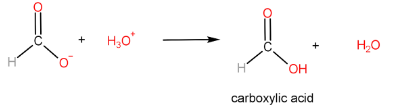
The reaction of carboxylic acid with a primary alcohol in the presence of sulphuric acid to form an ester is called esterification reaction.
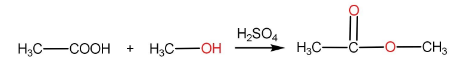
Step-I: Protonation of carboxylic acid to form carbocation
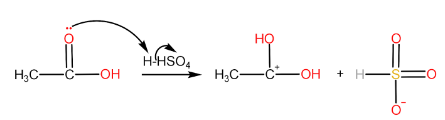
Step-II: Attack of alcohol on the carbocation followed by proton transfer within the molecule.
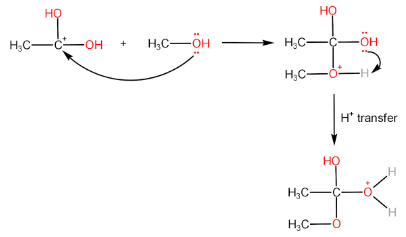
Step-III: Removal of H2O molecule.
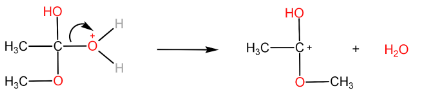
Step-IV: Removal of H+ ion by HSO4− to form ester and H2SO4
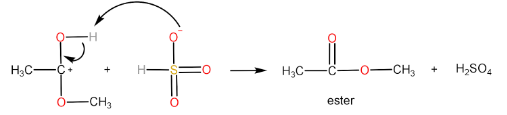
Decarboxylation is the removal of a carboxyl group thereby releasing CO2 usually from a carboxylic acid to form a product with one carbon less than the reacting carboxylic acid.
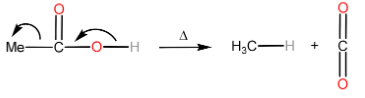
Note:
Mostly there is a confusion between the aldol condensation and the Cannizzaro reaction. We have to remember that in aldol condensation two aldehyde molecules having α−H react with each other in the presence of dilute base while in Cannizzaro reaction two aldehyde molecules which do not have α−H react in the presence of strong base.
