Question
Question: Explain the following: Tetravalency....
Explain the following: Tetravalency.
Solution
For solving this question, we need to understand the octet rule. As we know that a carbon atom has four electrons in its outermost valence shell. So, it needs four more electrons to complete its octet. For which it needs to form bonds with other atoms to get electrons and complete its octet.
Complete step by step answer:
As we know that the carbon is a periodic element present in the group 14 and having atomic number of 6 . The electronic configuration of carbon is represented by 1s22s22p2 which clearly shows that outermost four orbitals are half-filled. Thus it needs four electrons to form a stable compound.
So, the carbon atom forms four equivalent covalent bonds by sharing valence electrons with other atoms. This is known as tetravalency of carbon,(tetra means four). These four valences of carbon are directed towards four corners of a tetrahedron, and inclined to each other at an angle of 109.5∘ .
We must know that the methane has a tetravalency nature with molecular formula of CH4 and structure of methane is represented by ,
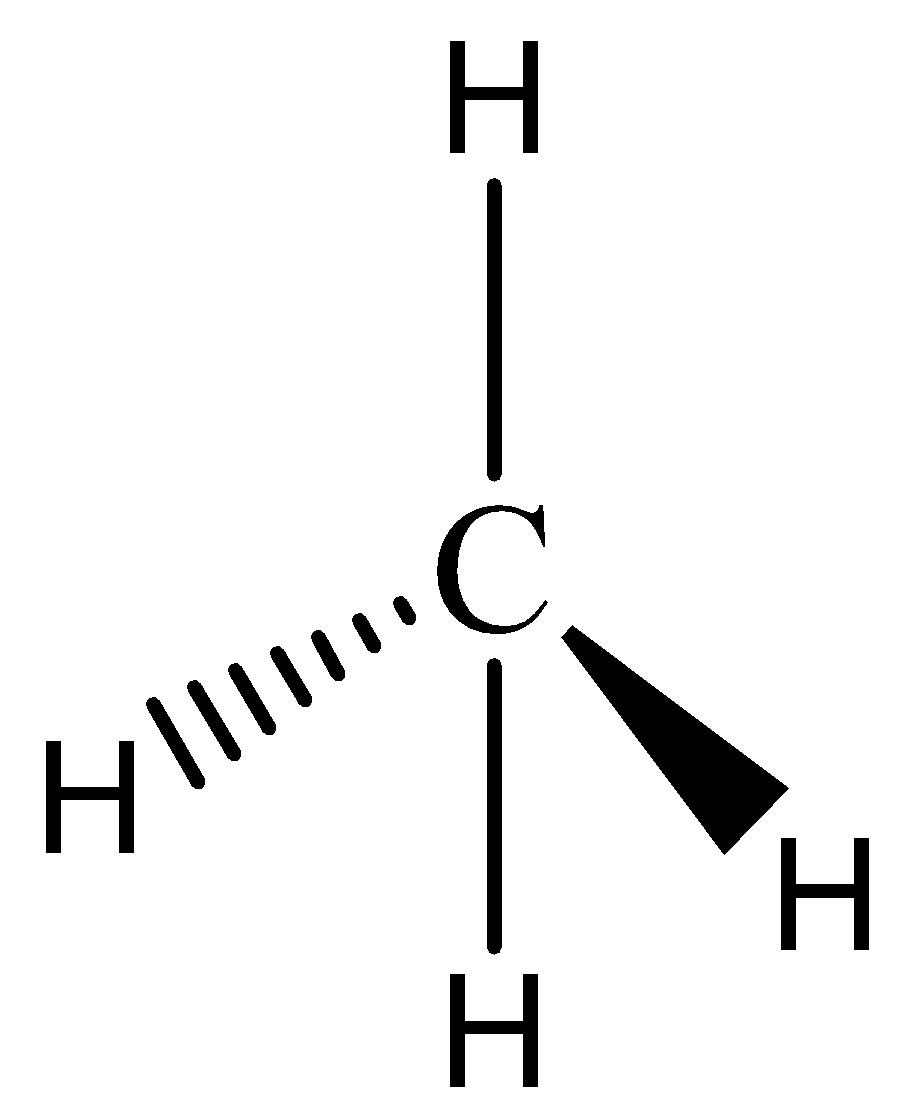
Here, we can see that the carbon atom is assumed to be the atomic center of the tetrahedron. The four valences of carbon are represented by four bonds that are around a carbon atom.
Also, we know that the total ionization energy required to remove four electrons from an atom is so high, that these elements do not form ionic compounds in their tetravalent state.
Note: We must remember that the no. of atoms present in an element which is replaced by another atom like hydrogen is known as valance. As we know that the noble gas present in group 18 in the periodic table has a fulfilled valence electron thus they are more stable than the other elements. We have to remember that in a chemical reaction a compound is formed by sharing valence electrons of the two atoms.
