Question
Question: Explain the following reactions i) Reimer-Tiemann reaction ii) Williamson synthesis...
Explain the following reactions
i) Reimer-Tiemann reaction
ii) Williamson synthesis
Solution
We need to know that the Reimer-Tiemann reaction is one of the important reactions in aromatic compounds. Karl Riemer and Ferdinand Tiemann discovered this reaction. Hence, this reaction is called the Reimer-Tiemann reaction.We have to remember that the Williamson synthesis is a naming reaction for the ether synthesis. It is the first reaction to prove the formation of ether and describe the structure of the ether. Ether is nothing but one oxygen is bonded in between two alkyl groups.
Complete step by step answer:
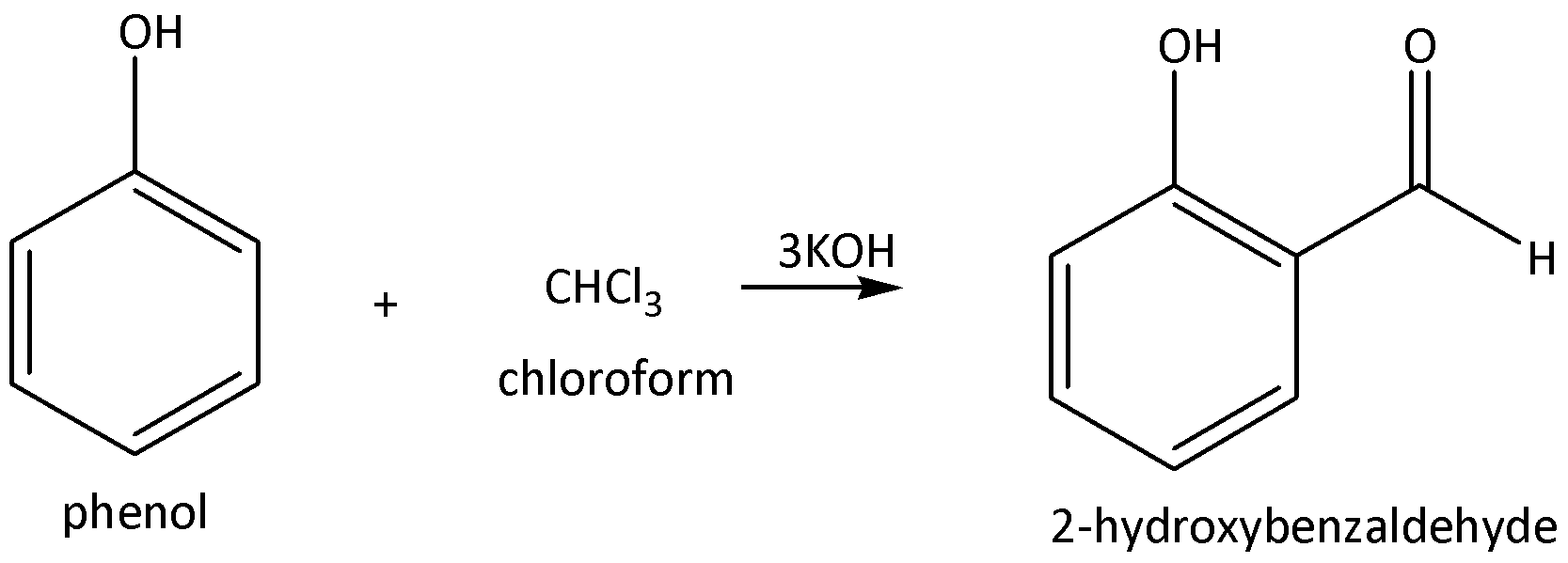
We need to remember that in the Reimer-Tiemann reaction, starting material is phenol. Phenol is nothing but one hydrogen in a benzene ring that is replaced by a hydroxyl group. Phenol reacts with the presence of chloroform, excess of potassium hydroxide is required for formation of Salicylaldehyde as a product. Salicylaldehyde is a common name and IUPAC name is 2-hydroxybenzaldehyde of the product. In this reaction selectively, to form the aldehyde group in ortho position in the ring.
We have to remember that in Williamson synthesis means one of the ether formation naming reactions. In this reaction, sodium alkoxides react with alkyl halide to form ether with sodium halide.
NaOR+R−Cl→R−O−R+NaCl
For example for Williamson’s synthesis, sodium ethoxide reacts with ethyl chloride to form diethyl ether with sodium chloride. We can write the chemical equation for this reaction as,
CH3CH2ONa+CH3CH2Cl→CH3CH2OCH2CH3+NaCl
Note:
We have to remember that in mono substituted benzene rings have two ortho positions, two Meta positions and one para position. Ortho position is nothing but the bond angle between the substituted group and the coming group is 60∘ . Meta position means bond angle between substituted and coming group is 120∘ .Para position means bond angle between substituted and coming group is 180∘ . Para position is directly opposite and ortho position is near to the mono substituted group. Williamson synthesis is one of the easiest ways to prepare ether in the laboratory. Sodium salt of alkoxides is a good reactant in Williamson synthesis compared to other salts.
