Question
Question: Explain the following named reaction: i)Sandmeyer reaction. ii)Gattermann reaction....
Explain the following named reaction:
i)Sandmeyer reaction.
ii)Gattermann reaction.
Solution
The name reactions are those reactions which are named after its discoverers, or developers. The above name reactions are used for the formation of aryl halide from diazonium salts.
Complete step by step answer:
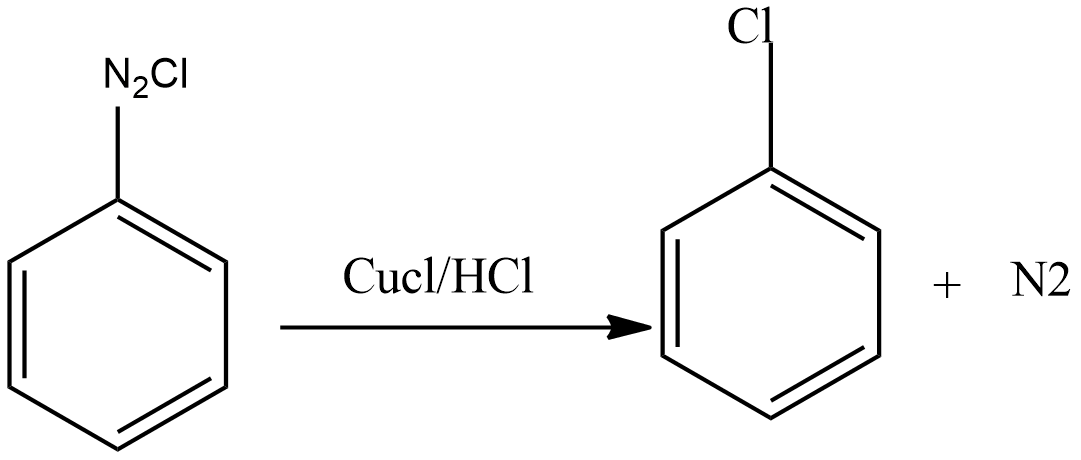
Sandmeyer reaction: In this reaction when diazonium salts are treated with CuCl dissolved in HCl or CuBr dissolved in HBr then formation of aryl chloride or aryl bromide takes place.
The sandmeyer reaction is written as:
In the first step we form nitrous acid from sodium nitrite.
NaNO2+HCl(dil)→HONO+NaCl
Then nitrous acid reacts with aniline to form diazonium salt.
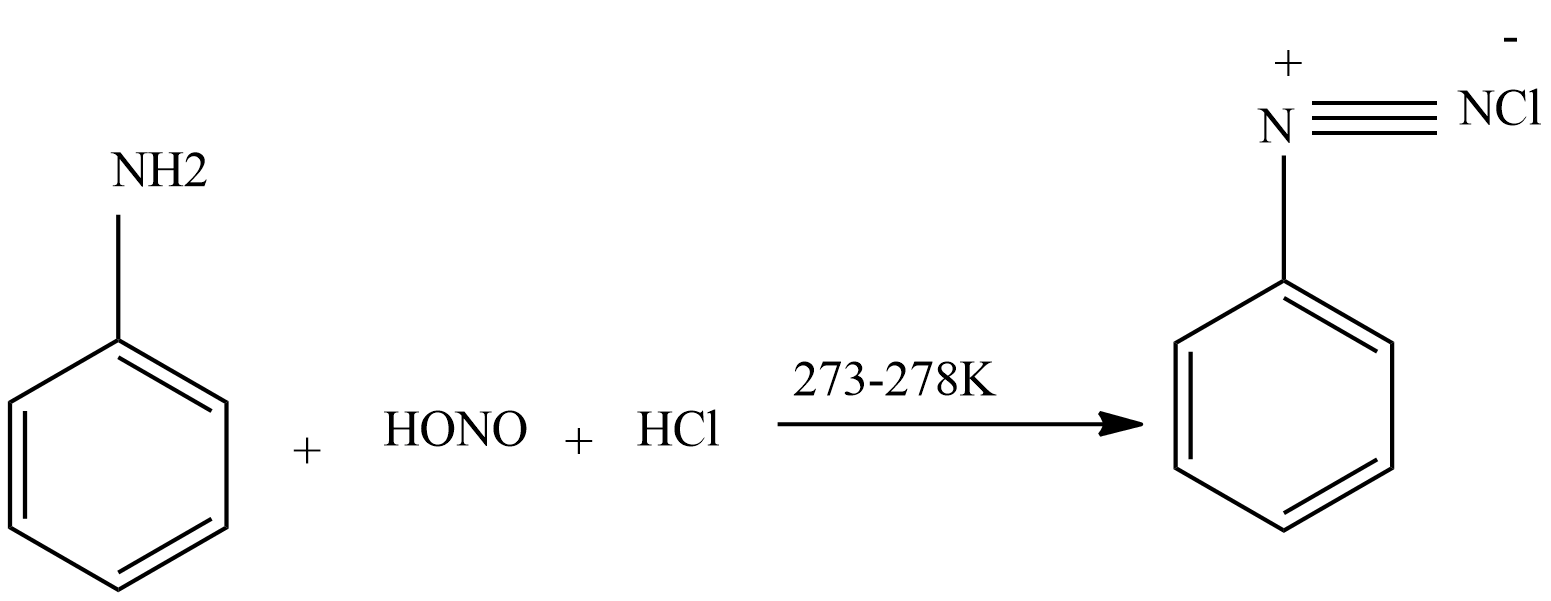
Then diazonium salt reacts with CuCl dissolved in HCl to form chloroarenes.
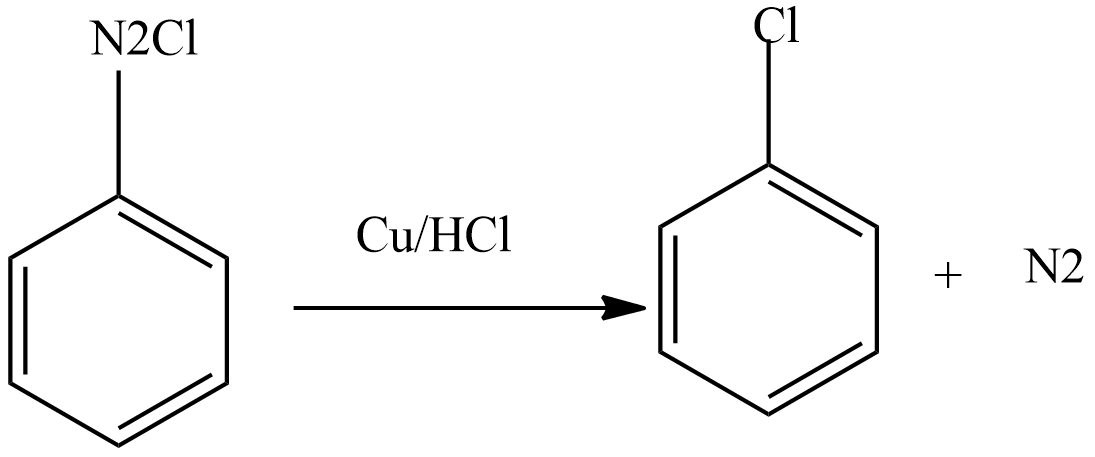
Gattermann reaction: This reaction is the modification of the sandmeyer reaction. In this reaction, a mixture of freshly prepared copper powder in the presence of halogen acid is used instead of cuprous halide dissolved in corresponding halogen acid.
First in this reaction also we make nitrous acid from sodium nitrite.
NaNO2+HCl(dil)→HONO+NaCl
Then again nitrous acid reacts with aniline to form diazonium chloride.
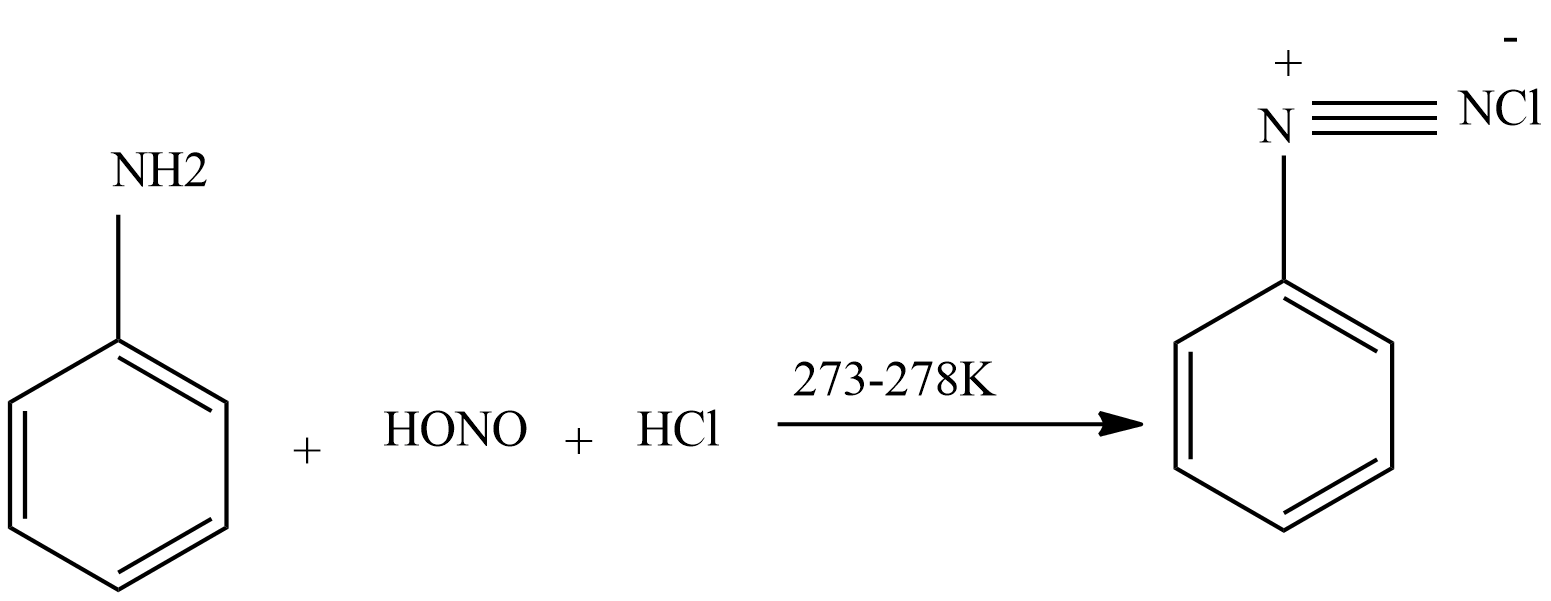
Then diazonium chloride reacts with freshly prepared copper powder in the presence of halogen acid to form aryl halide.
Note:
-Sandmeyer reaction is the two step mechanism reaction. The Sandmeyer reaction follows the free radical mechanism. In this reaction, the halogen attached to copper enters the benzene ring.
-The yield obtained by the Gattermann reaction is 40%.
