Question
Question: Explain the effect of the inductive effect on the reactivity of alkyl halides....
Explain the effect of the inductive effect on the reactivity of alkyl halides.
Solution
Halogen group is an electron-withdrawing group because it requires only one electron to become stable. So it acquires a negative charge and the alkyl group will acquire a positive charge.
Complete step by step solution:
The displacement of σ−electrons along the saturated carbon chain whenever an electron-withdrawing or electron-donating group is present at the end of the carbon chain is called inductive effect or I-effect.
–I-Effect is when the substituent attached to the end of the carbon chain is electron-withdrawing.
+I-Effect is when the substituent attached to the end of the carbon chain is electron-donating.
So, when the atom or group like halogen which is an electron-withdrawing group attached to the alkyl carbon chain, the σ−electrons of C−X are attracted by or displaced to the more electronegative atom i.e., halogen atom. Due to which the halogen atom will acquire a small negative charge and the carbon atom of the alkyl group will acquire a small positive charge.
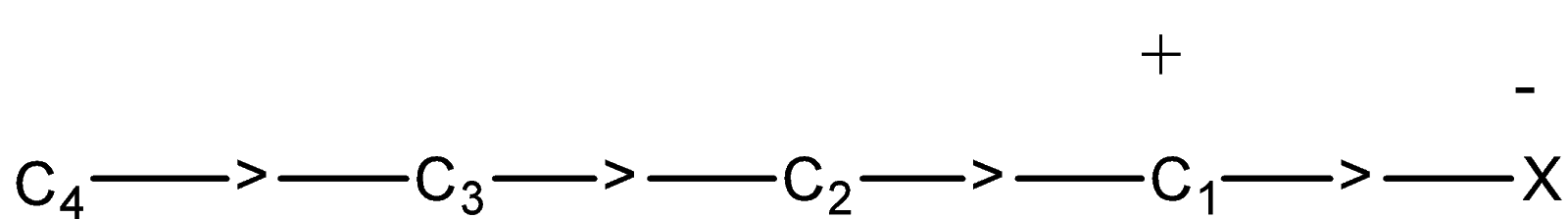
Now in this chain, there is a positive charge on C1 atom, this, in turn, attracts the σ−electrons of C1−C2 bond towards it. Due to this C2 will also have some positive charge and this, in turn, will attract the electrons of C2−C3 bond towards it. Due to this C3 will also acquire a very small positive charge.
So, the reactivity of alkyl halide due to the inductive effect is that the alkyl part acts as an electron-donating group and the halide acts as an electron-withdrawing group.
Note: The same process occurs when an electron-donating group is attached to the alkyl group, but the difference is that the alkyl group will acquire the negative charge and the electron-donating group will acquire the positive charge.
