Question
Question: Explain the difference between 1,2- and 1,3- chelating amines? Show example....
Explain the difference between 1,2- and 1,3- chelating amines? Show example.
Solution
Chelating agents are chemical compounds that react with metal centres to form a stable complex. They are also known as chelates or chelators. Chelating agents form at least two bonds with the metal centres and have a ring-like structure. Chelating imines are the ligands that have two imine groups.
Complete Step By Step Answer:
First, we will explain what 1,2-diamines are. A common example of 1,2-diamines are bis-(dimethyl glyoximato)nickel (II). The 1,2-diimine ligand is the dimethylglyoximato ligand and bis indicates the presence of 2 similar ligands in the complex. The chemical formula is Ni(DMG)2 .
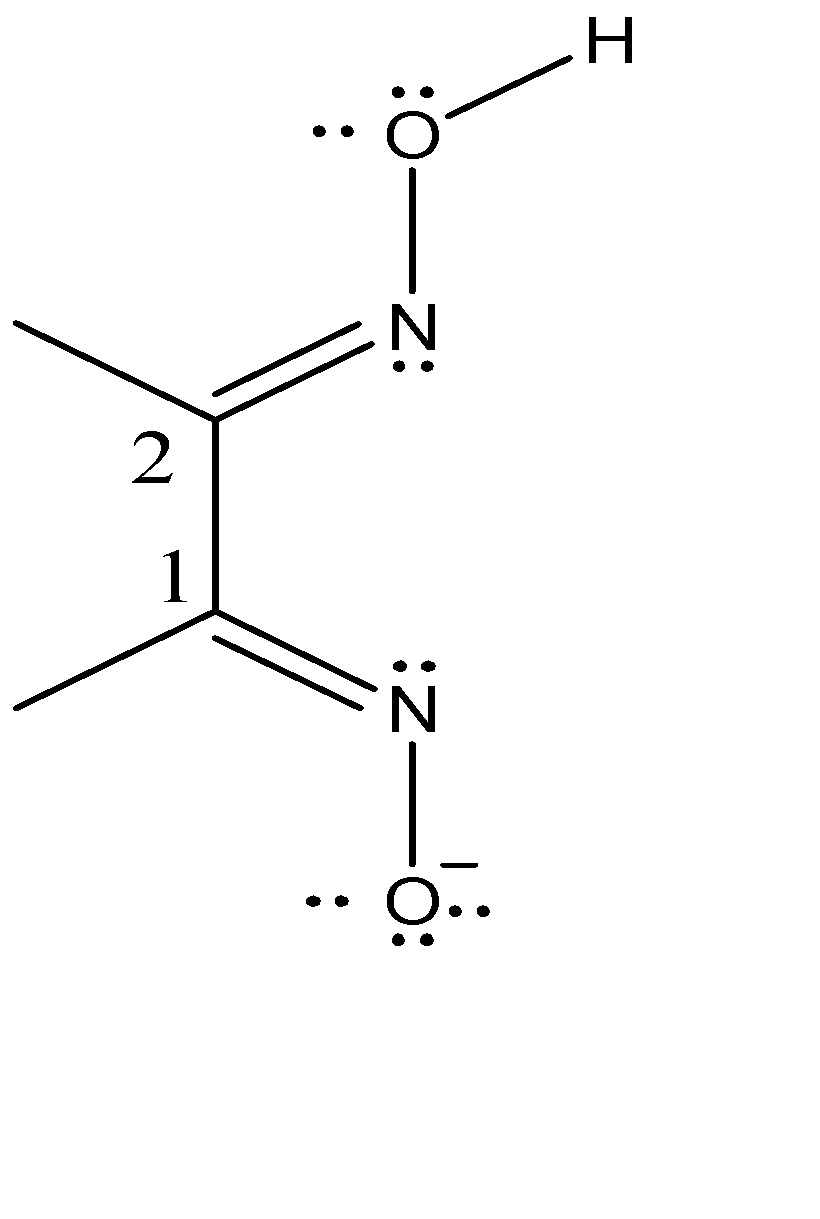
If we consider the imine carbons are ‘Carbon-1’ then the second imine carbon (Carbon-2) is one carbon away. Hence, we name it as 1,2-diimine. Dimethyl glyoximato ligand is abbreviated as DMG and has the net charge of -1. Each nitrogen in the ligand is the binding site due to the presence of lone pairs of electrons. Since the binding of more than one ‘tooth’ is entropically favoured, hence this two ’toothed’ binding requires the binding sited to be cis. Therefore, the DMG ligand prefers cis binding.
Next is 1,3-diimine ligands, the common example of which is N,N’-diphenyl-2,4-pentanedionato ligands. The common example of this ligand is dichloro(nacnac)nickel(II). The chemical formula is [Ni(nacnac)Cl2] . The nick name of this ligand is simple ‘nacnac’. Nacnac is also a bidentate ligand with two nitrogen being the binding sites, using their lone pair of electrons. Here, the immune carbons are two carbons away, hence the name 1,3-diimines.
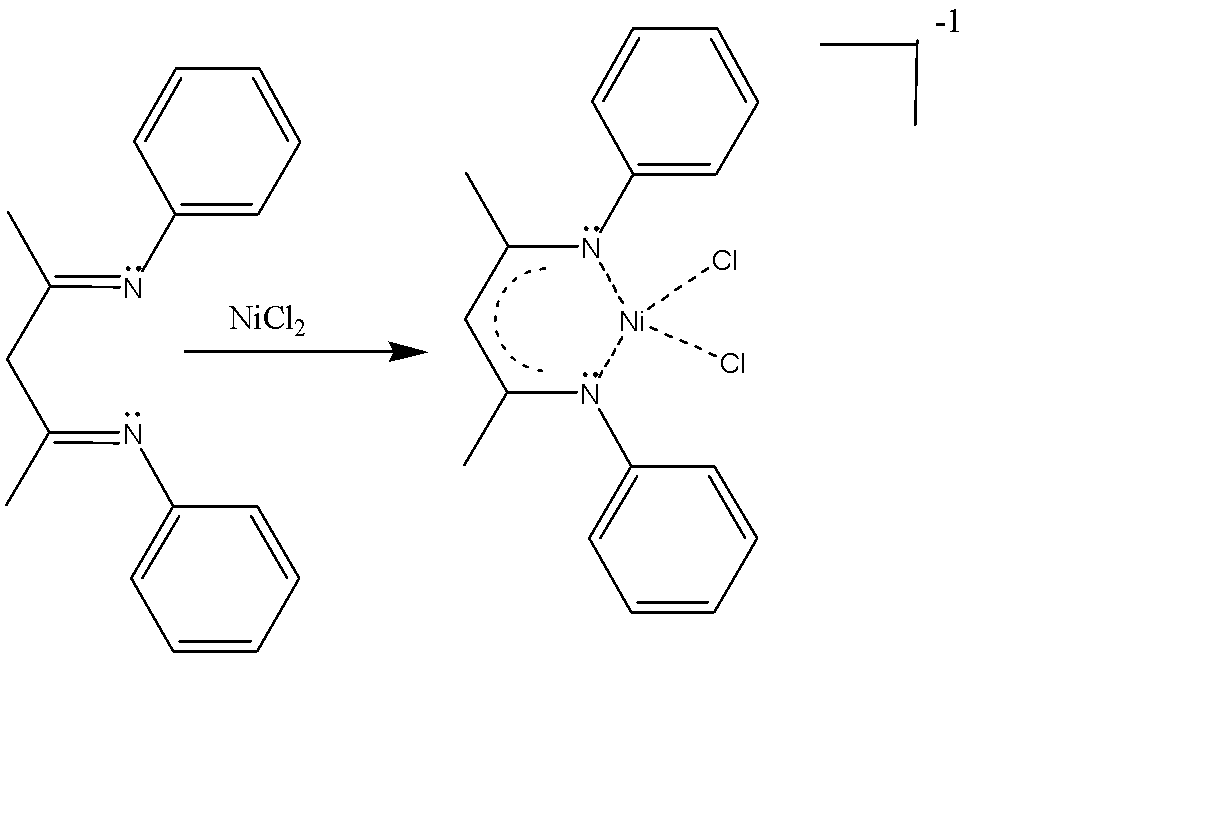
The net charge on the complex is -1 and favours cis binding due to chelate effect favouring the binding of both teeth rather than only one.
Note:
Substituted diamine ligands are useful for the preparation of post-metallocene catalysts for the polymerization and copolymerization of ethylene and alkenes. They also act as precursors to NHC ligands, by the condensation with formaldehyde.
