Question
Question: Explain the bonding in methane molecules using electron dot structure....
Explain the bonding in methane molecules using electron dot structure.
Solution
The chemical formula of methane molecule is CH4. It contains four carbon-hydrogen sigma bonds.
Complete answer:
A covalent bond is formed when two atoms share one electron each. Axial overlap of two orbitals form a sigma bond. A covalent sigma bond contains a pair of electrons.
The atomic numbers of hydrogen and carbon are 1 and 6 respectively. The electronic configurations of hydrogen and carbon are 1s1 and 1s22s22p2 respectively. Thus, the number of valence electrons for hydrogen and carbon are 1 and 4 respectively. To attain stability hydrogen should have 2 electrons in its valence shell. For this purpose, a hydrogen atom shares one electron with carbon atom, the carbon atom also shares one electron with this hydrogen atom. Thus, a carbon-hydrogen covalent bond is formed. To attain stability, the carbon atom needs eight electrons in its valence shell. For this purpose, the carbon atom shares 4 electrons with 4 hydrogen atoms to form 4 carbon-hydrogen covalent bonds.
You can write the following structure for methane molecules.
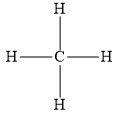
You can write the following electron dot structure for methane molecules.
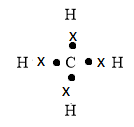
In the above structure, the dot represents the electron of a carbon atom and the cross represents the electron of hydrogen atom.
Note: Since the central carbon atom in methane molecule has 4 bonds and zero lone pairs of electrons, it will undergo sp3 hybridization to form four degenerate sp3 hybrid orbitals. These four degenerate sp3 hybrid orbitals of carbon overlap with four 1s atomic orbitals of 4 hydrogen atoms to form four carbon-hydrogen covalent bonds. Methane molecules have tetrahedral geometry.
