Question
Question: Explain the Acidic nature of phenols and compare with that of alcohols....
Explain the Acidic nature of phenols and compare with that of alcohols.
Solution
Hint: If a compound can donate protons, then it is called acidic. Phenol and alcohol donate its electron to form an ion each. One of the ions is very stable.
Complete step by step solution:
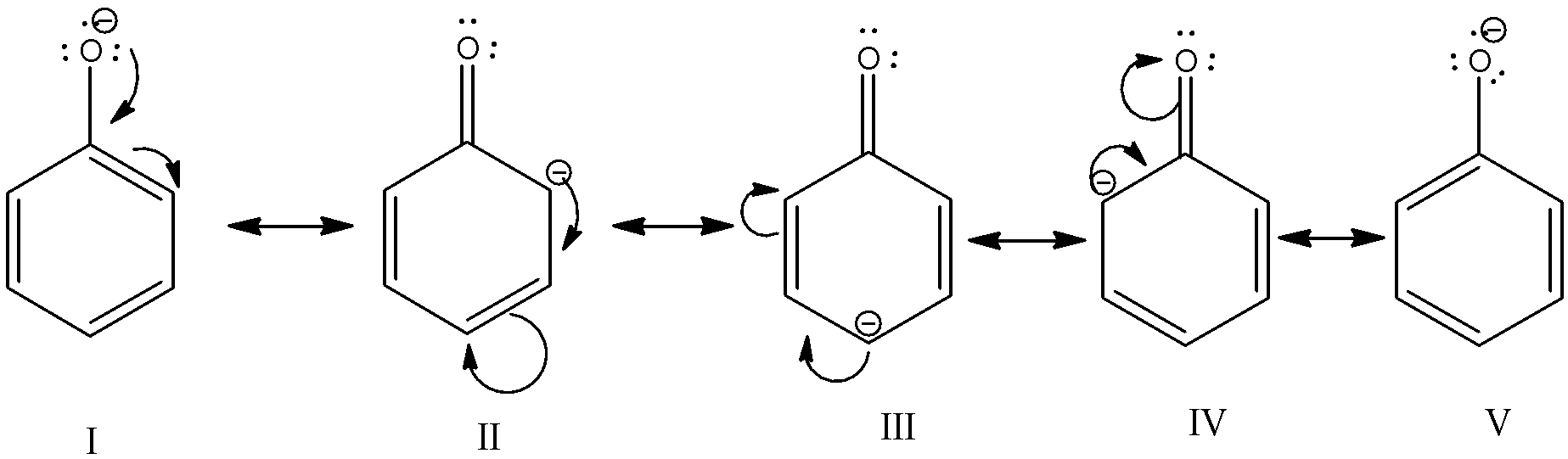
-Acidic character of a compound means its ability to release protons when it is dissolved in water. In case of phenols, when it is dissolved in water, it dissociates to give a phenoxide ion and a proton. This phenoxide ion is highly stable due to resonance. The oxygen of phenoxide ion has 3 lone pairs and a negative charge due to the loss of a proton. This negative charge on oxygen is involved in resonance with double bonds of benzene rings. Five resonance structures are formed. We know that, if a structure is accompanied by resonance, then it is highly stable. Same is the case here. Phenoxide ion formed is very stable. So, phenol easily gives up the proton so that it can form phenoxide ions. Thus, it is acidic.
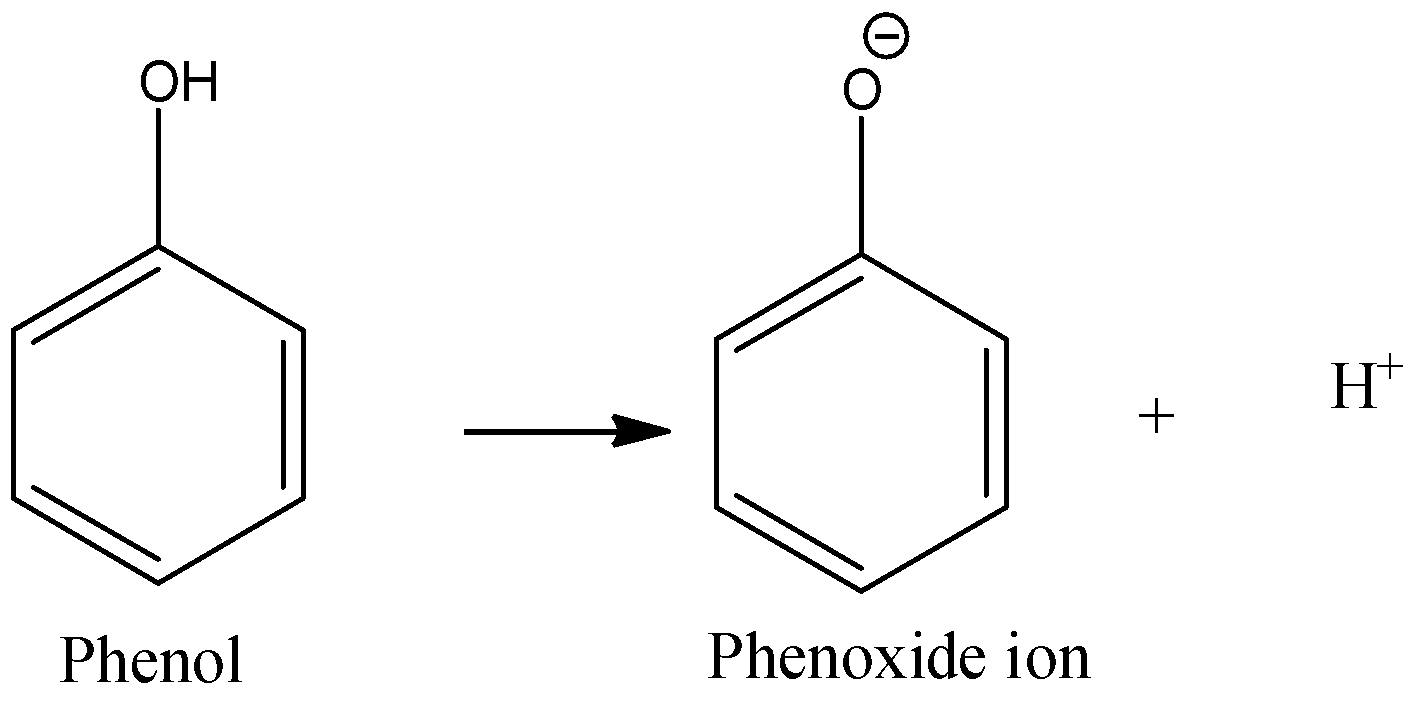
-When an electron withdrawing group is present, it increases the acidity of phenol by stabilizing the phenoxide ion, while if an electron releasing group is present; it destabilizes the ring and the acidity of phenol decreases. Phenol is still a weak acid.
-In case of alcohols, alkyl group is attached to the oxygen atom. Oxygen is electron releasing in nature because of the inductive effect (+I). So, oxygen attains a partial positive character and it cannot lose hydrogen in alcohol, that is its tendency to donate protons decreases. It is very less acidic than phenol.
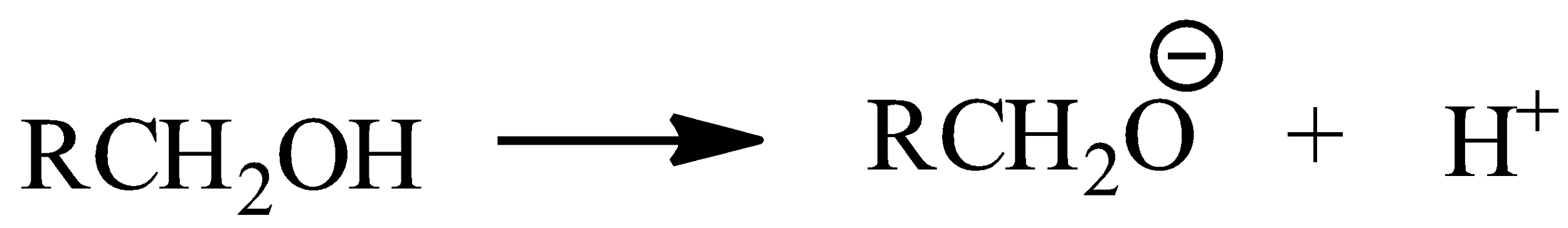
Since, phenols can easily donate protons rather than alcohols, phenols are more acidic than alcohols.
Note: In phenols, the OH group is connected to a stable benzene ring. In alcohols, the OH group is not directly connected to aromatic or a benzene ring; usually it is some alkyl group.
