Question
Question: Explain \({{\rm{S}}_{\rm{N}}}1\) reaction with appropriate example....
Explain SN1 reaction with appropriate example.
Solution
SN1 reaction is one of the substitution reactions in organic chemistry. It is a basic reaction which is favoured by polar protic solvents. Polar protic solvents refer to the solvents possessing high value of dipole moment and dielectric constant.
Complete step by step answer:
The SN in the reaction name suggests that it is a nucleophilic substitution and the one suggests that the rate determining step is unimolecular. The process involves a nucleophile that replaces a leaving group. These reactions are unimolecular. In this reaction, the rate only depends on the concentration of one reactant. It is a two-step SN2 is faster than SN1. Because SN1 is a single step mechanism.
One example of SN1 reaction is,
(CH3)3CBr+−OH→(CH3)3COH+Br−
We know that SN1reaction takes place in two steps. Let’s understand the mechanism of the above reaction.
In step I, cleavage of C−Br bond takes place to produce a carbocation and a bromide ion.
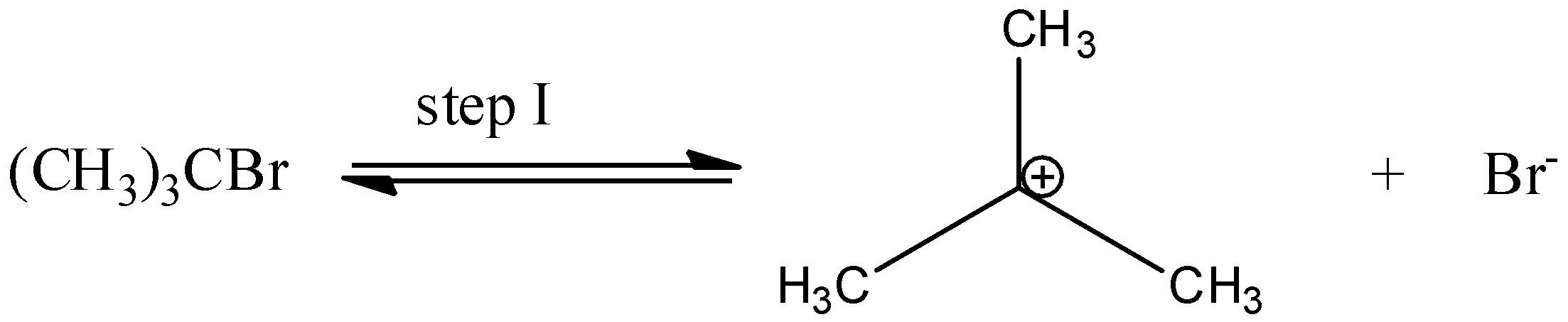
In the 2nd step a nucleophile attacks the carbocation to form alcohol.
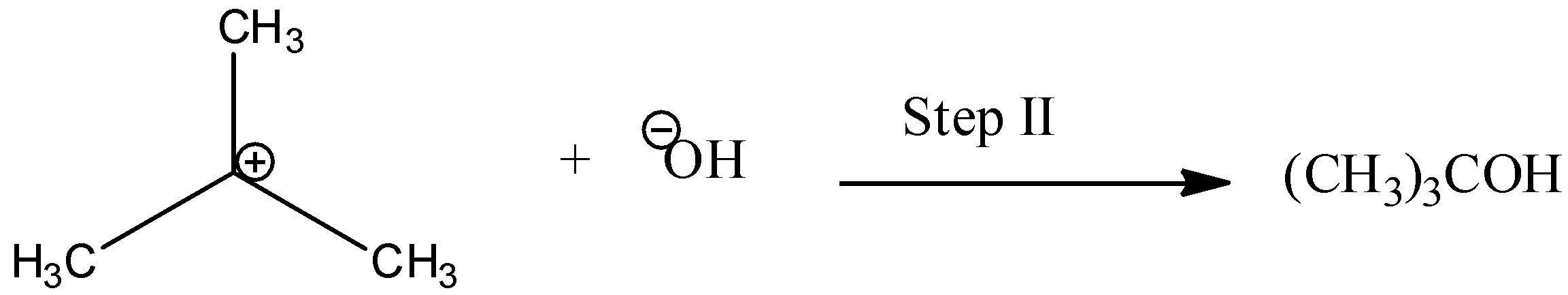
The step I is the reversible and slowest step. It involves the breaking of C−Br for which energy is obtained by salvation of the halide ion with the proton of protic solvent. We know that the rate of reaction depends only on the slowest step. So, rate is dependent on concentration on alkyl halide.
Note: Factors that affect rate of SN1 reaction:
The rate can also depend on the strength of the incoming nucleophile.
The rate of the reaction can be increased by using highly polar solvent.
SN1 reactions generally describe a bond breaking process whereas SN2 reactions describe a bond making process. SN1 reactions are unimolecular and SN2 reactions are bimolecular in rate of the reaction.
