Question
Question: Explain ion-dipole interaction with suitable examples....
Explain ion-dipole interaction with suitable examples.
Solution
The result of electrostatic interaction between a charged ion and a molecule that has a dipole is nothing but, ion-dipole interaction. It is an attractive force that is commonly found in solutions, especially ionic compounds dissolved in polar liquids.
Complete step by step solution:
-An ion-dipole interaction occurs between a fully charged ion and a partially charged dipole. The strength of the ion-dipole force is proportionate to ion charge.
-An ion-induced dipole interaction occurs between a fully charged ion and a temporarily charged dipole. The temporary dipole is induced by the presence of the ion.
-Ion-dipole and ion-induced dipole forces operate much like dipole-dipole and induced dipole-dipole interactions. However, ion-dipole forces involve ions instead of solely polar molecules.
-Ion-dipole forces are stronger than dipole interactions because the charge of any ion is much greater than the charge of a dipole; the strength of the ion-dipole force is proportionate to ion charge. Ion-dipole bonding is also stronger than hydrogen bonding. An ion-dipole force consists of an ion and a polar molecule aligning so that the positive and negative charges are next to one another, allowing for maximum attraction.
-Ion-dipole forces are generated between polar water molecules and a sodium ion. The oxygen atom in the water molecule has a slightly negative charge and is attracted to the positive sodium ion. These intermolecular ion-dipole forces are much weaker than covalent or ionic bonds.
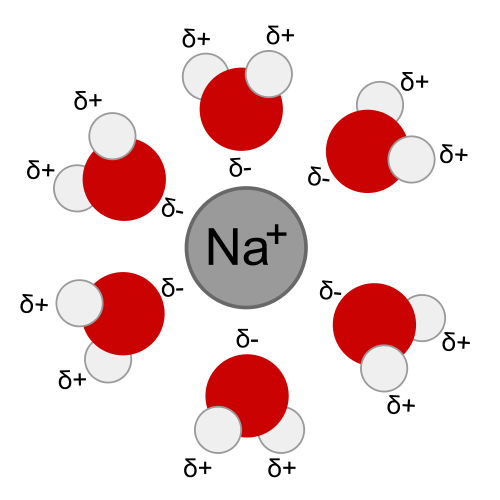 -Ion-dipole forces are generated between polar water molecules and a sodium ion. The oxygen atom in the water molecule has a slightly negative charge and is attracted to the positive sodium ion. These intermolecular ion-dipole forces are much weaker than covalent or ionic bonds.
-Ion-dipole forces are generated between polar water molecules and a sodium ion. The oxygen atom in the water molecule has a slightly negative charge and is attracted to the positive sodium ion. These intermolecular ion-dipole forces are much weaker than covalent or ionic bonds.
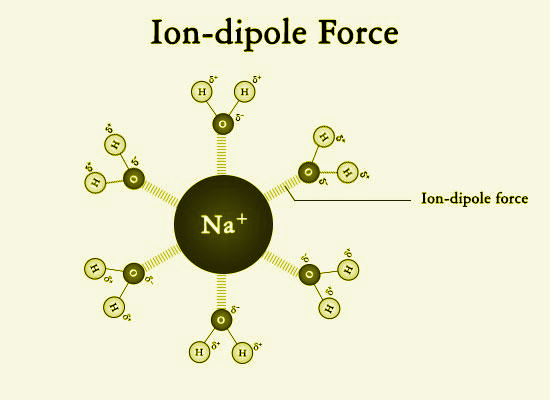
Note: Ion-dipole is mainly found in the polar molecules. But, when this type of force is found in non-polar molecules, this is called ion-induced dipole forces. An ion-induced dipole force occurs when an ion interacts with a non-polar molecule. Like a dipole-induced dipole force, the charge of the ion causes a distortion of the electron cloud in the non-polar molecule, causing a temporary partial charge. The temporary partially charged dipole and the ion are attracted to each other and form a fleeting interaction.
