Question
Question: Explain in detail how furan is aromatic?...
Explain in detail how furan is aromatic?
Solution
Aromatic compounds are cyclic and have planar structure accompanied by delocalized π-electron clouds in place of individual alternating double and single bond have high stabilization energies and are very stable.
Complete step by step answer:
To understand how furan is aromatic we need to understand
Huckel Rule
According to this rule of cyclic system must contain (4n+2)π− electron used in delocalization (including lone pair), where n is any positive integer (0,1,2,3,..........)
Furan:
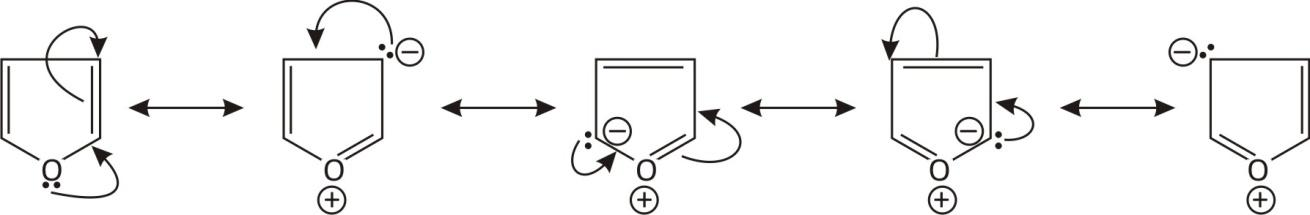
If we look at the structure of furan it contains two double bonds and oxygen contains one lone pair of electrons.
When a lone pair of e− of oxygen resonates if forms a double bond with carbon and oxygen becomes +ve charged because it contains three bonds. And the carbon containing lone pair becomes negatively charged. This phenomenon is called Resonance. And form five resonance structures.
Now by looking at the structure we can conclude that these structures are cyclic planar and have resonating bonds. So Furan is an aromatic compound.
Huckel Said if a molecule with these properties have (4n+2)π electrons that molecule is considered as aromatic
In Furan (4n+2)=6πe− (in this 4πe−and 2e− form lone pair)
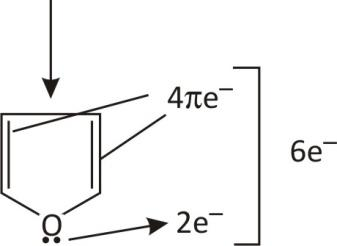
(here n= no of ring)
Lone pairs are contributing in resonance
Hence furan follows huckel rule and is an aromatic compound.
Note: To decide aromaticity value of n should be known and π electrons used in delocalisation.
Aromatic compounds are less reactive than alkenes, making them useful industrial solvents for non polar compounds.
The aromaticity order depends on the electronegativity of the heteroatom
Aromatic compounds have high carbon to hydrogen ratio.
