Question
Question: Explain hybridization of central atom in \(Si{H_4}\)...
Explain hybridization of central atom in SiH4
Solution
We can solve this question using steric number rule which is given as-
Steric number of an atom= number of atoms bonded with that atom+ number of lone pair left on that atom. So if the steric number is 2 then hybridization is sp and geometry is linear. If the steric number is 3 then hybridization is sp2 and geometry is trigonal planar. If the steric number is 4 then hybridization is sp3 and geometry is tetrahedral. If steric number is 5 then hybridization is sp3d and geometry is Trigonal bipyramidal. If steric number is 6 then hybridization is sp3d2 and geometry is octahedral.
Complete step by step answer:
Silicon has atomic number 14 and its electronic configuration is [Ne]3s23p2 . So it needs four electrons to complete its octet. InSiH4, the silicon atom is the central atom which forms four sigma bonds with hydrogen atoms. Since all the valence electrons participate in hybridization then it has no lone pair of electrons in it. So on using the formula of steric number rule which is given as-
⇒ Steric number of an atom= number of atom bonded with that atom+ number of lone pair left on that atom
Here, the number of hydrogen atom bonded with silicon =4 and number of lone pair left on silicon=0
On putting these values, we get-
⇒ Steric number of an atom= 4+0=4
Now we know that, if the steric number is 4 then hybridization is sp3 and geometry is tetrahedral. So the structure is given as-
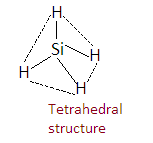
The hybridization of silicon in SiH4 is sp3 and geometry is tetrahedral.
Note:
We can also solve this question using this formula to calculate steric number–
⇒ 2No. of valence electrons + No. of monovalent atoms attached
Here we know that silicon has 4 valence electrons and the number of monovalent atom (hydrogen) is 4
Then on putting the values in the formula we get-
⇒24+4=4
Since steric number is 4 then hybridization is sp3 and geometry is tetrahedral.
