Question
Question: Explain hybridization of central atom in \[PC{l_5}\]...
Explain hybridization of central atom in PCl5
Solution
We need to find hybridization of a given molecule. We can find the hybridization by knowing the number of sigma bonds and lone pairs present on the central atom.
Complete step by step answer:
Hybridization is the concept of mixing atomic orbitals into new hybrid orbitals with different energies, shapes etc, that are suitable for the pairing of electrons to form chemical bonds in valence bond theory.
z=no.ofσbond+l.poncentralatom
Where, No. of σbond means single bond and l.p is the lone pair present on the central atom.
| Z | Hybridisation |
|---|---|
| 2 | Sp |
| 3 | Sp2 |
| 4 | Sp3 |
| 5 | Sp3d |
The phosphorus has atomic number 15 and valence electrons that are present are 5. These five electrons present, will make bonds with five Cl that are there in PCl5.There is no lone pair present.
The Hybridisation of PCl5 will be Sp3d as z is equal to five and corresponding geometry will be trigonal bipyramidal. There will be two types of bonds that will be formed in PCl5 that are equatorial bonds and axial bonds.
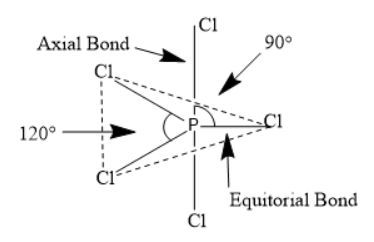
The structure which we can see has trigonal bipyramidal geometry and three chlorine atoms are present at the corner of a triangle whereas two chlorine atoms present are at axial position.
Equatorial bonds: There will be three P−Cl equatorial bonds which will lie in a single plane to make an angle with each other. The angle between the P−Cl bond will be 120∘.
Axial bonds: There will be two P−Cl axial bonds where one bond will lie above the equatorial plane and other will lie below the plane.. The angle between the P−Cl bond will be 90∘.
Note: The axial bonds will always be longer than the equatorial bonds as axial bonds are nearer to equatorial bonds so it experiences greater repulsion from equatorial bonds. As phosphorus is -Sp3d hybridization and it forms five equivalent Sp3d orbitals which each contain an unpaired electron and results in the formation of trigonal bipyramidal geometry.
