Question
Question: Explain Hund’s rule with an example....
Explain Hund’s rule with an example.
Solution
It is a rule discovered by the Friedrich Hund in chemistry, in order to set a regular pattern for filling of electrons in the orbitals of subshells of different shells present around the nucleus.
Complete step by step answer:
According to the Hund’s rule if many degenerate orbitals are present then no one of the all orbitals will contain two electrons initially until all orbitals was occupied by one electron i.e. whenever more than one orbitals are present which are having same energy then firstly they all should be occupied by one electron with same spin and after this half-filled condition orbitals may start pairing of electrons.
For example: If five orbitals are present and we have to fill 4 electrons in these 5 orbitals. So, as we know that each orbital will have the capacity to carry 2 electrons in it, but according to the hund’s rule firstly we have to fill all the orbitals with a single electron who have the same spin and then we start pairing singly occupied orbitals.
If we filled electrons like above is given then that will be wrong:
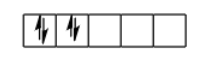
And if filled electrons according to the Hund’s rule then that will be correct & shown as follow:
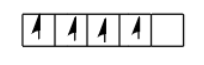
Note: Here some of you may make mistakes in the filling of electrons if different orbitals are given, but always keep in mind that any kind of orbitals are present first you have to fill the orbitals with a single electron only and then start pairing.
