Question
Question: Explain how the properties of iron can be changed by the addition of carbon?...
Explain how the properties of iron can be changed by the addition of carbon?
Solution
As carbon atoms are larger than the iron atoms, so when they go inside the interstices of the crystal lattice of iron, imperfections are created and the iron becomes harder.
Complete answer:
In order to answer our question, we need to learn about impurity defects. Foreign atoms can occupy interstitial or substitutional sites in a crystal. Defects in the ionic solids can be found out by adding impurity ions. If the impurity ions have different valence states than that of the host ions, vacancies are created. For example, addition of SrCl2 to NaCl yields solids solutions where each Sr2+ ion replaces two Na+ ions. Sr2+ion occupies the site of one Nation whereas the other site remains vacant. Thus, it produces cation vacancies equal to the number of the Sr2+ ions occupying substitutional sites.
In the metallic crystal of iron, the atoms are held very tightly to each other by application of strong metallic bonds. So, in order to deform the crystal structure, a lot of force needs to be applied. However, there are some vacancies created in the crystal structure of iron.
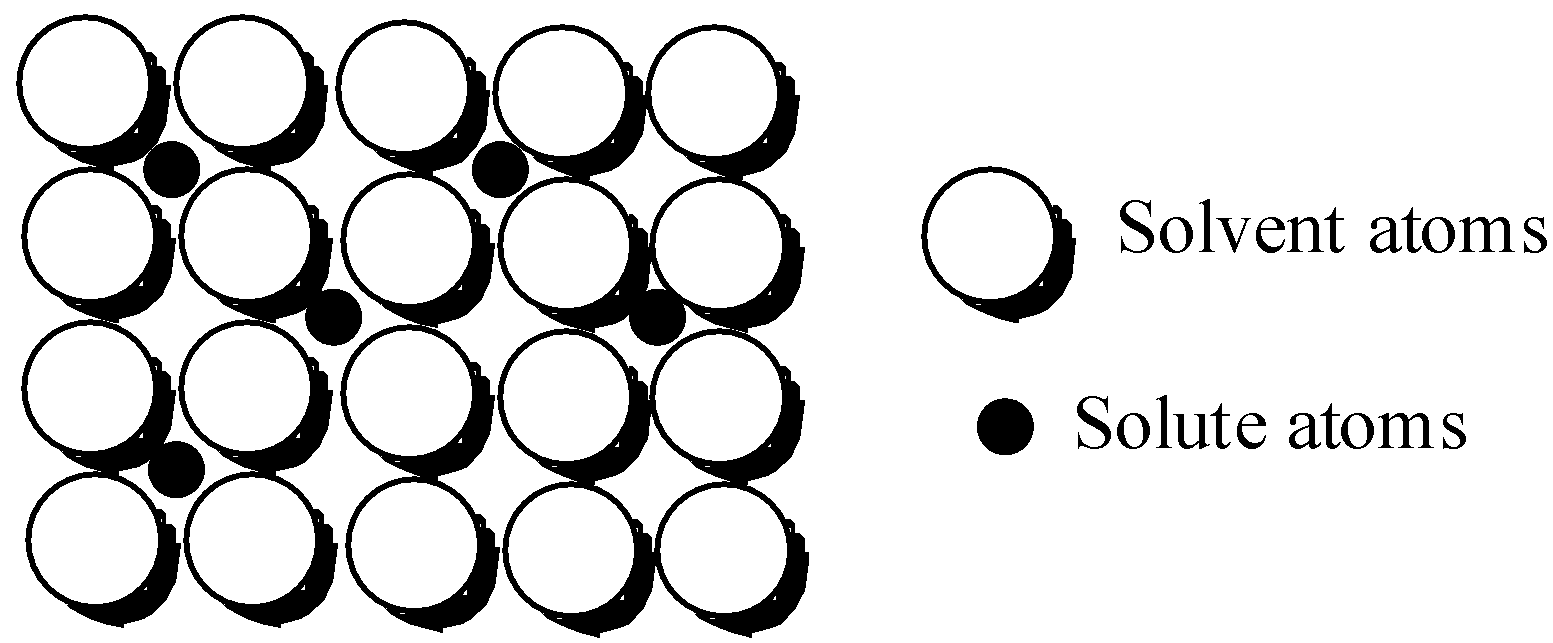
But, the carbon atom is larger in size than the iron atom, so it is larger to fit inside the perfectly arranged interstices of the crystal lattice. As a result, a lot of imperfections are created and no more vacant space is left out. Iron is relatively soft, but after introducing carbon, the layers do not slide past each other easily and this makes the iron harder.
Note:
The process of introducing an external element in a metal crystal lattice to improve its properties is called doping.
