Question
Question: Explain Hall-Heroult method of obtaining pure aluminium from alumina with figure....
Explain Hall-Heroult method of obtaining pure aluminium from alumina with figure.
Solution
This is an industrial process to obtain pure aluminium. It involves the melting of aluminium oxide also known as oxide in molten cryolite and electrolysing the molten salt bath. This process takes place at 940−980∘C and produces 99.5−99.8% pure aluminium.
Complete answer:
Charles Hall and Paul Heroult invented a method to obtain aluminium from alumina with the help of electrochemical methods. The melting point of alumina is very high and passing of electric current does not take place through solid form of alumina, therefore a small amount of cryolite is added to carry the electrolysis. This acts as a good conductor of electricity. When we add feldspar it lowers the melting point of the alumina. So in all a mixture of alumina, cryolite, and feldspar is subjected to electrolysis to obtain the pure form of aluminium. For the process of electrolysis, an iron vessel where we can see a coating on the inside of this vessel of carbon layered graphite. This acts as a cathode. This carbon rod is connected with a copper clam and immersed in an electrolyte. This acts as an anode. When electric current is passed, the molten aluminium will be deposited at the cathode and there will be release of oxygen at anode.
The process can be understood by the following diagram:
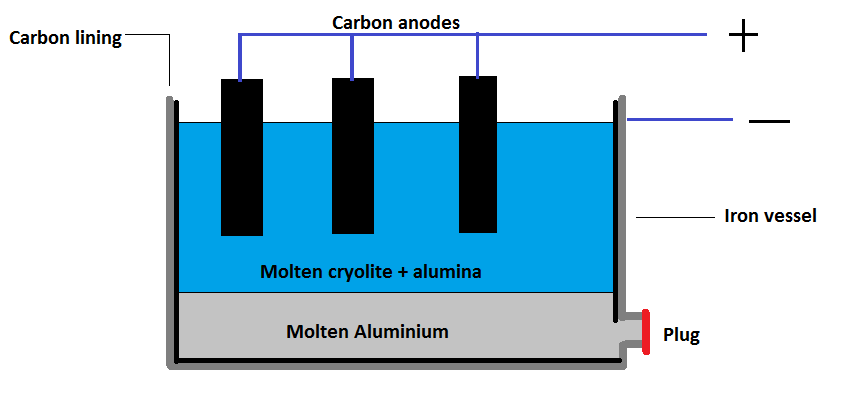
The reaction is given as follows;
At cathode- 2Al3+(I)+6e→2Al(I)
At anode- 6O2−(I)→6O2(g)+12e−
Note:
Elemental aluminium is not possible to be obtained by electrolysis of an aqueous aluminium salt, because hydronium ions oxidize elemental aluminium. Molten aluminium salt can be used instead of aluminium oxide and has a melting point of 2072∘C .
