Question
Question: Explain, giving reasons for your answer, why Calcium is more reactive than Magnesium but less reacti...
Explain, giving reasons for your answer, why Calcium is more reactive than Magnesium but less reactive than Sodium?
Solution
In order to solve this question, we should know about reactivity series. The reactivity series is the arrangement of elements according to their reactivity. It is also known as the activity series .It also tells about the element which oxidizes more rapidly to form positive ions.
Complete Step By Step Answer:
The reactivity series is the series in which elements are arranged according to their reactivity, how fast they react. The more reactive elements are placed on the top while least on the bottom. More reactive element loses electrons greatly and forms positive ions. Whereas the least reactive element loses electrons slowly as compared to the highly reactive element. The reactivity series of the element is:
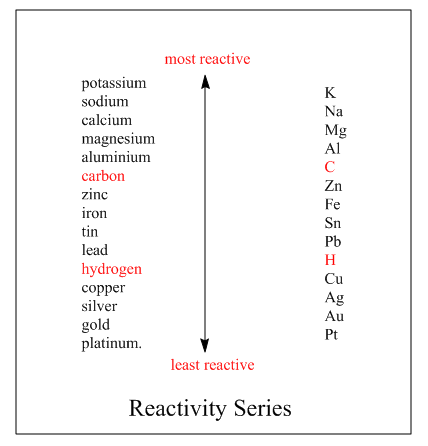
On moving up the series the reactivity of the elements increases, thus the element becomes more reactive. Here Potassium is the most reactive element than other as it is on the top of the series whereas Platinum on the bottom, thus least reactive element than others. As from series we can see calcium is above the magnesium in the reactivity series. Hence it is more reactive than magnesium. On the other hand, it is below the sodium in the reactivity series, so it is less reactive than sodium.
Note:
The reactivity series also tells about the elements which corrode or tarnish more rapidly, also require more energy to be isolated from their compounds and which element can become stronger reducing agents.
