Question
Question: Explain \({{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\) is an inner orbital complex whereas \({{[Ni{{(N{{H}_{3...
Explain [Co(NH3)6]3+ is an inner orbital complex whereas [Ni(NH3)6]2+ is an outer orbital complex.
Solution
The ligand and the number of electrons in the valence orbitals of the metal are going to decide the complex is inner orbital or outer orbital complex. To form an inner orbital complex the ligand should be a strong ligand.
Complete answer:
- In the question it is asked to explain [Co(NH3)6]3+ is an inner orbital complex whereas [Ni(NH3)6]2+ is an outer orbital complex.
- Coming to the complex [Co(NH3)6]3+ .
- In this complex cobalt in +3 oxidation.
- The electronic configuration of cobalt is 1s22s22p63s23p64s23d7 .
- The electronic configuration of Co3+ is 1s22s22p63s23p64s03d6 .
- We can write the electron representation in Co3+ as follows.
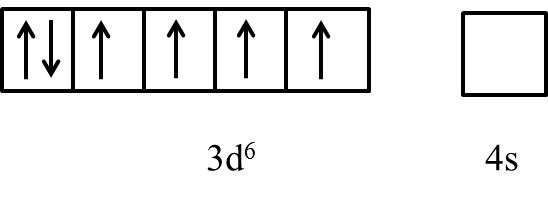
- In the presence of the ligand like ammonia the electrons in the 3d orbital are going to pair up and it is as follows.
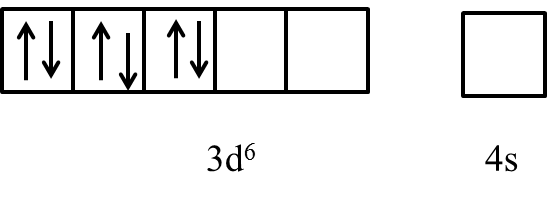
- After pairing the electron in the 3d orbital of the cobalt the last two orbitals are ready to participate in the formation of the bond with ligands and form an inner orbital complex with a hybridization of d2sp3 .
- Coming to [Ni(NH3)6]2+ .
- In this complex Nickel is in +2 oxidation.
- The electronic configuration of Nickel is 1s22s22p63s23p64s23d8 .
- The electronic configuration of Ni2+ is 1s22s22p63s23p64s03d8 .
- We can write the electron representation in Ni2+ as follows.
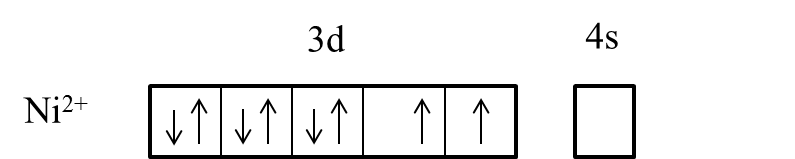
- In the presence of the ligand like ammonia the electrons in the 3d orbital are going to pair up and it is as follows.
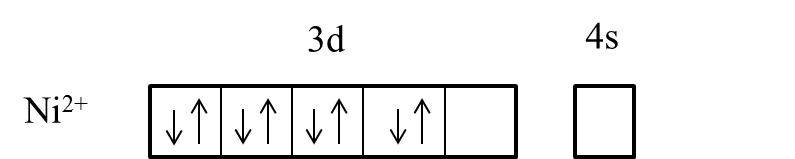
- After pairing the electrons in the 3d orbital of the Nickel, it has only one empty to participate in the hybridization.
- But it is not possible to participate only one 3d orbital in the hybridization, therefore nickel undergoes sp3d2 hybridization and forms an outer orbital complex with ligand ammonia.
Note:
There is an availability of d-orbitals in the process of hybridization to form inner orbital complexes. If there is no vacant 3d orbital in the metal then the metal undergoes outer orbital complex with the respective ligands.
