Question
Question: Expanded octet can be observed in the valence shell of the central atom in: A. \(N{H_3}\) B. \(C...
Expanded octet can be observed in the valence shell of the central atom in:
A. NH3
B. CH4
C. PCl5
D. BeCl2
Solution
We can say transfer of electrons as a process where an electron shares one or more electrons to its nearby atom. We know that there must be eight electrons in the outermost orbital of an atom. This is known as the octet rule. If an atom contains less than eight electrons, they have the ability to react and produce stable compounds.
Complete step by step answer:
We have to remember that the NH3 has six electrons in its bond pair and there is one lone pair (two electrons) present in nitrogen, so a sum of eight electrons are obtained. We can draw the structure of this compound as,
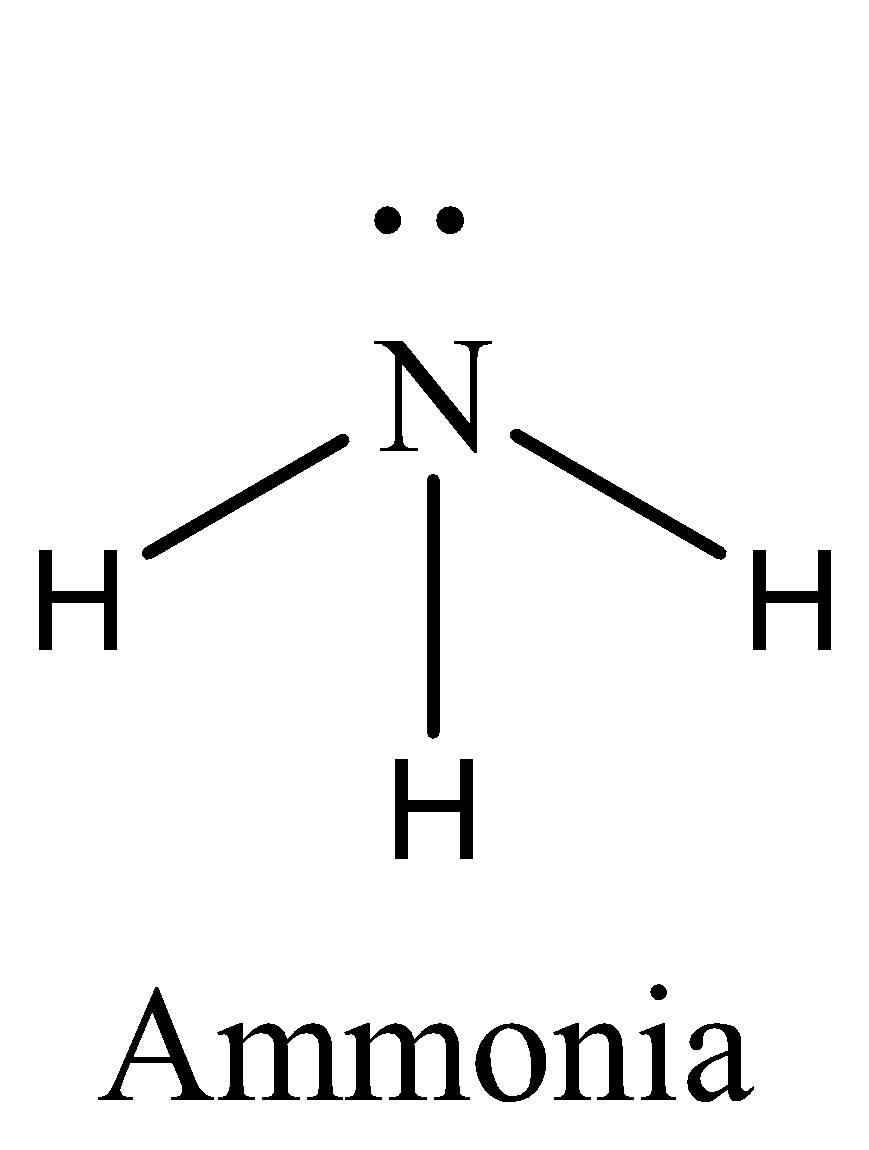
So NH3 does not have an expanded octet. Therefore, the option (A) is incorrect.
As we know that the CH4 has eight electrons in its bond pair and there is no one lone pair present in carbon, so a total of eight electrons are obtained. We can draw the structure of this compound as,
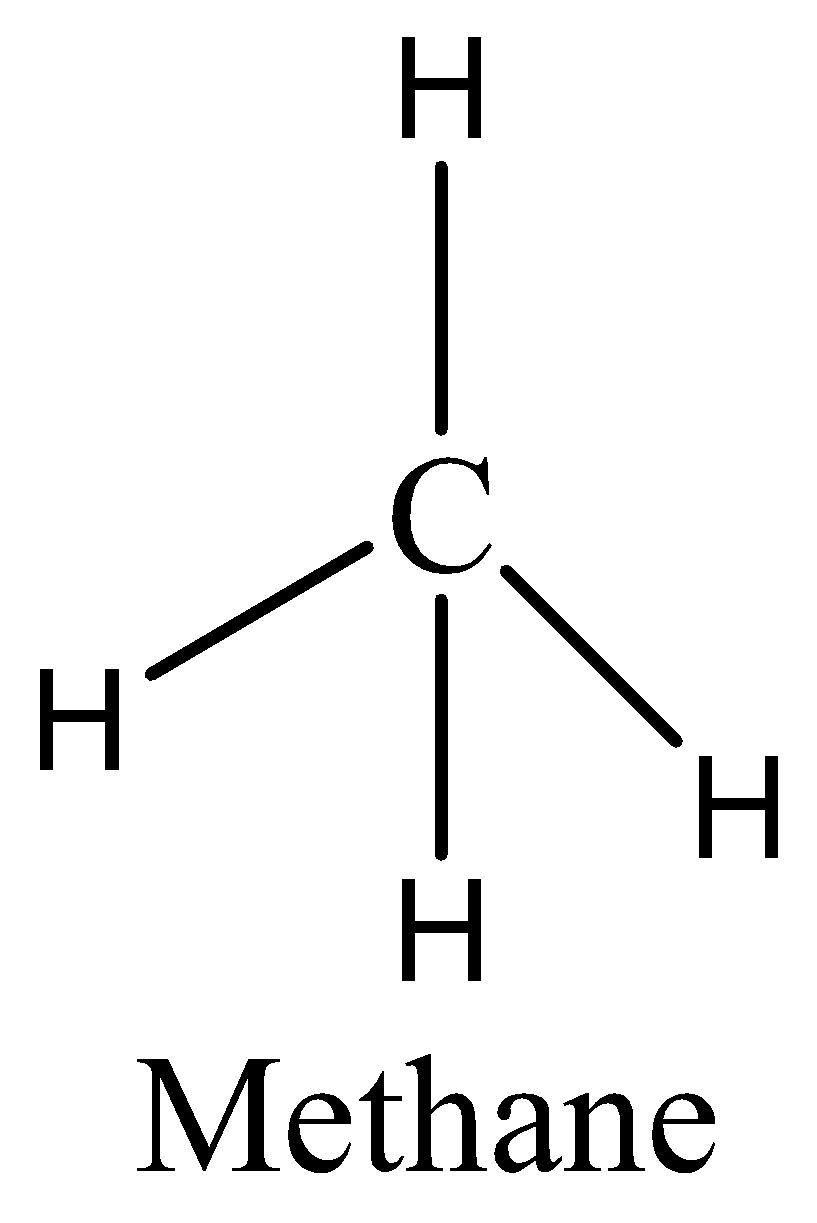
So CH4 does not have an expanded octet. Therefore, the option (B) is incorrect.
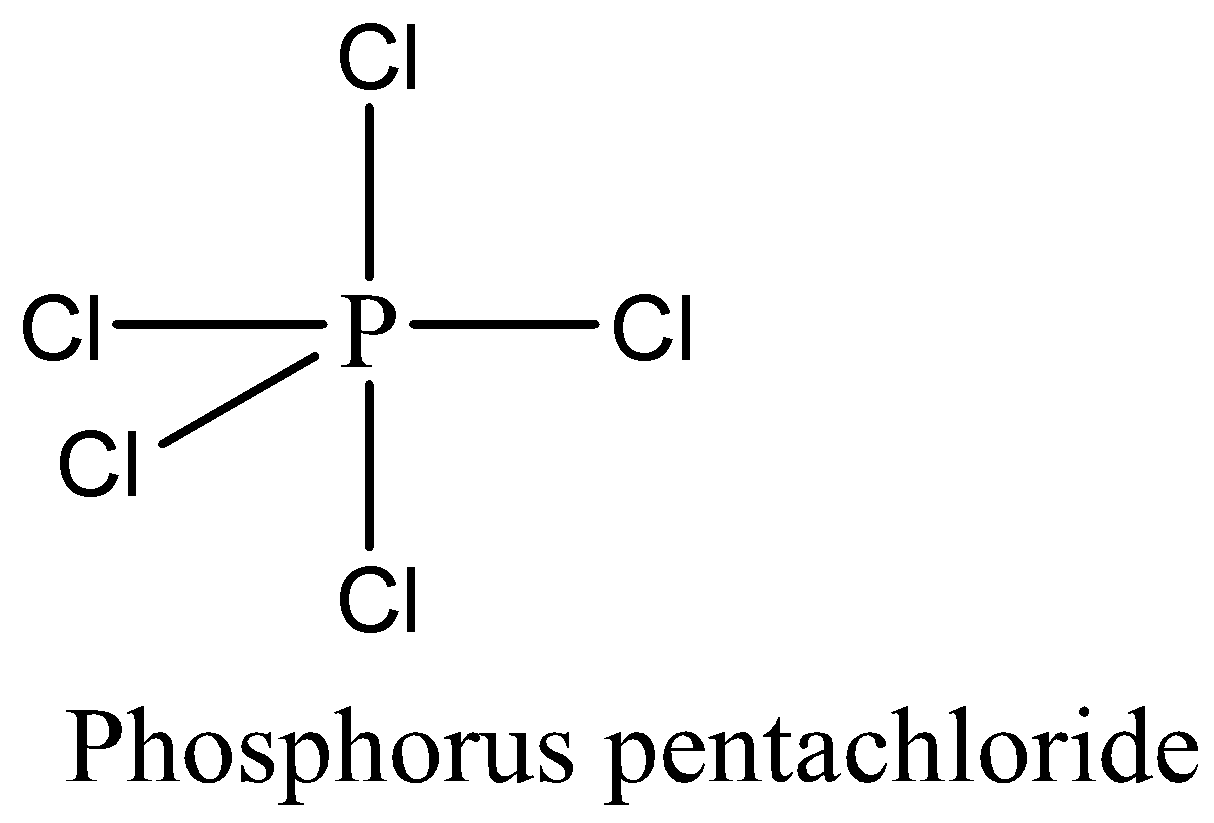
We have to remember that the PCl5 has 10 numbers of bonding electrons by sharing 5 valence electrons of phosphorus with 5 chlorine atoms and no lone pair of electrons are present. The expanded octet rule is satisfied by PCl5.
Therefore, the option (C) is correct. We can draw the structure of this compound as,
We have to remember that the BeCl2 does not show expanded octet, beryllium atoms join with each atom of chlorine through a single bond. Instead of an octet, the outermost shell of beryllium has two electrons pairs. They do not have any complete octet.
Therefore, the option (D) is incorrect. We can draw the structure of this compound as,
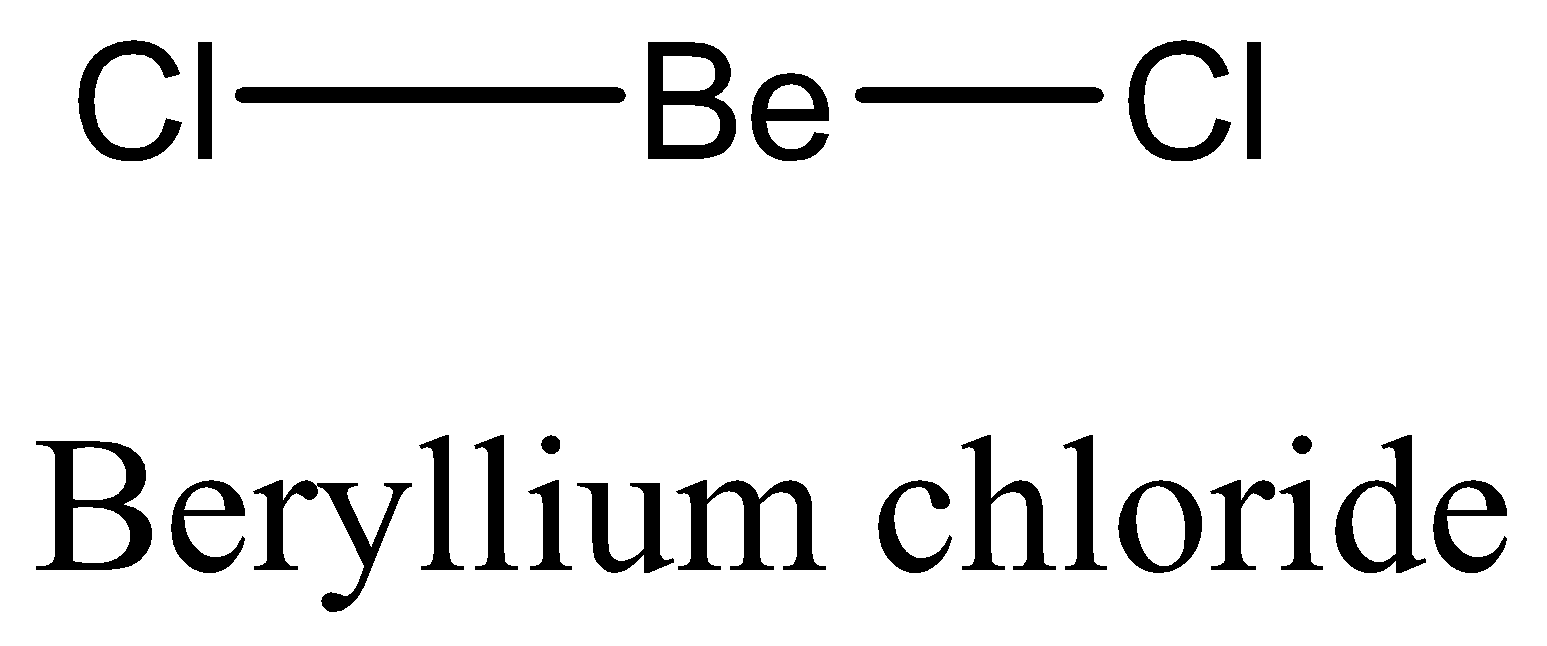
So, the correct answer is Option C.
Note: We have to remember that in SF6 the atom that does not obey octet rule is sulfur. The central sulfur atom gives six covalent bonds to six fluorine atoms. So it is an expanded valence shell molecule. The atom of sulfur expands its octet, hence the molecule SF6 interrupts the octet rule. In BH3 the atom which differs from the octet rule is boron. There are only six valence electrons in BH3 around the central atom boron. The atom of boron exhibits incomplete octet and so, the molecule BH3 disturbs the octet rule.
