Question
Question: Expand each of the following bond-line formulas to show all the atoms including carbon and hydrogen:...
Expand each of the following bond-line formulas to show all the atoms including carbon and hydrogen:
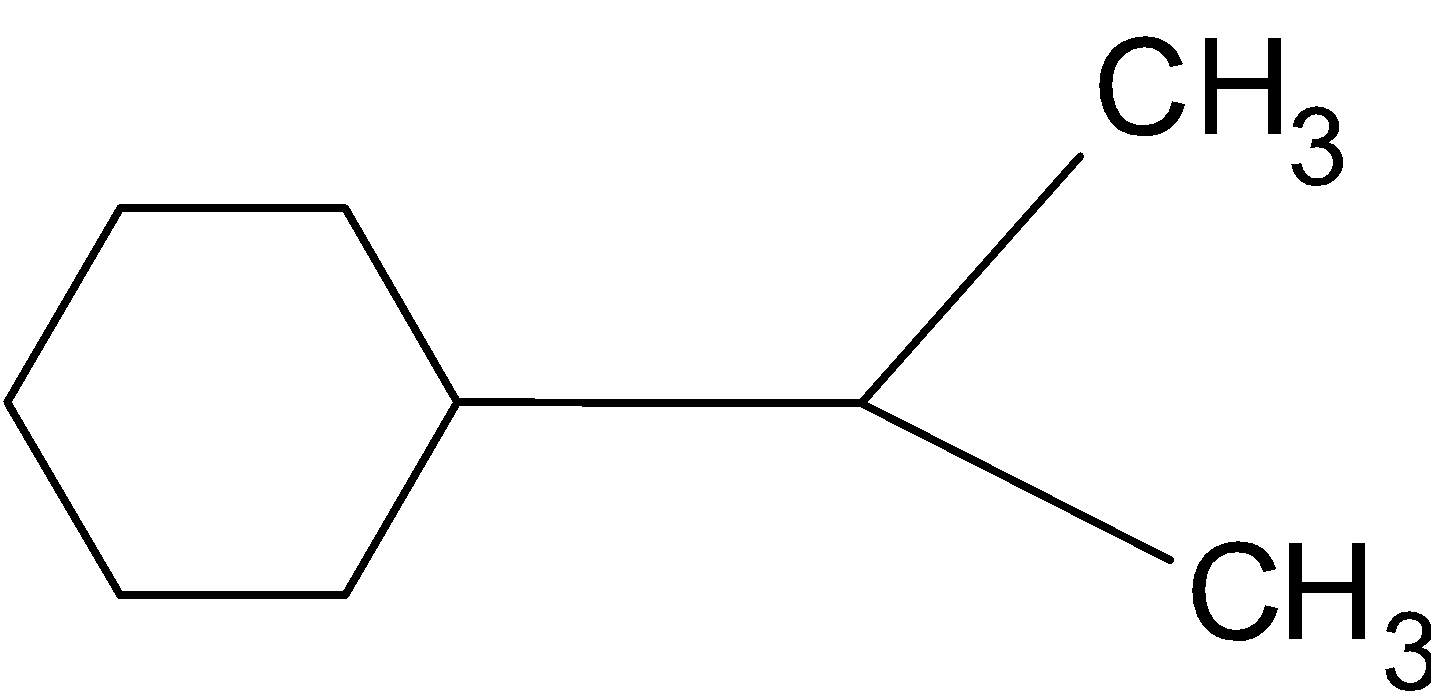
Solution
Hint: We know that each carbon can share 4 electrons to make bonds, so each carbon can make for bonds. These bonds can be single bond, double bond or triple bond. But in the case of cyclic compound triple bonds are not possible.
Step by step solution:
Bond-line structure (bond-line formula, skeletal structure, skeletal formula): A representation of molecular structure in which covalent bonds are represented with one line for each level of bond order.
We know that each hydrogen can make a single bond or can share a single electron. In a cyclic hydrocarbon structure each carbon is attached with a minimum two carbon atoms. This given compound is a cyclic compound which means one or more series of atoms in the compound is connected to form a ring. Below given is the bond – line formula of given structure: 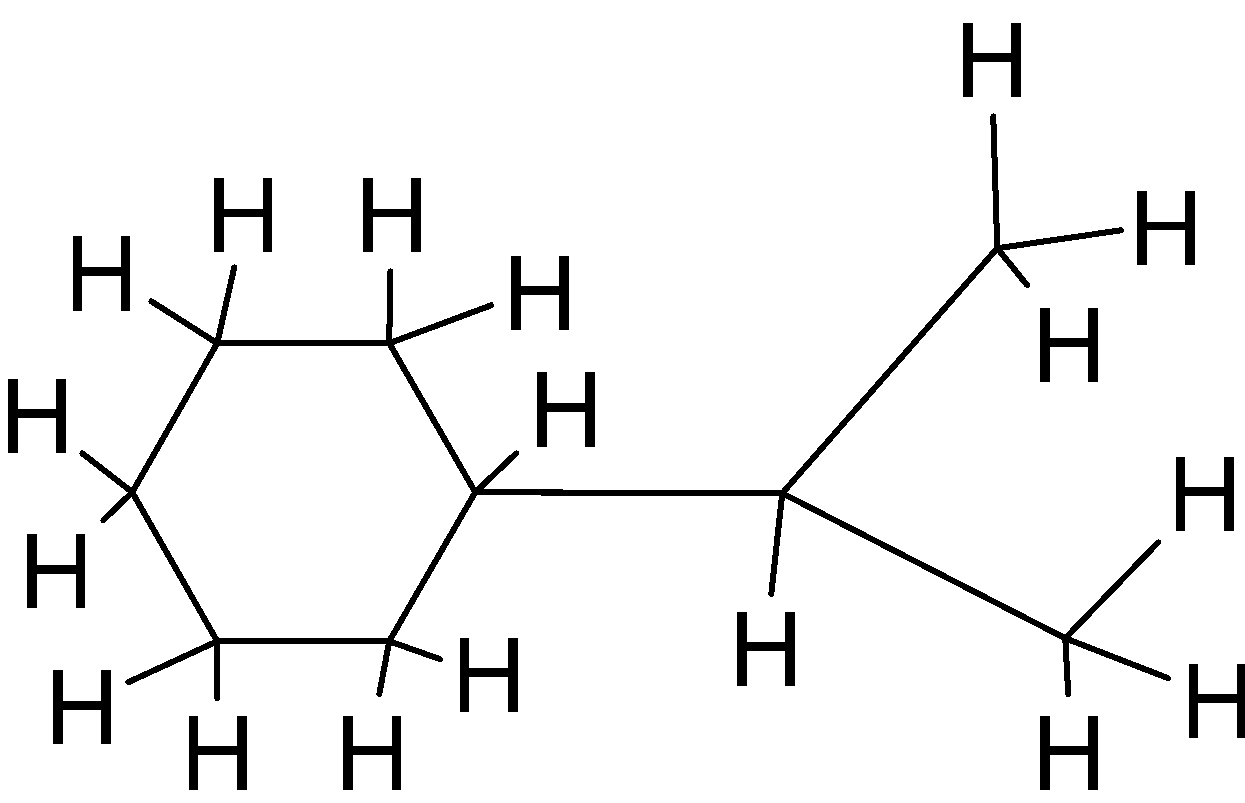 Note: Don’t make mistakes in making bonds of carbon. There should be four bonds made by each carbon and one bond by each hydrogen. And bond line and Lewis structures are different.
Note: Don’t make mistakes in making bonds of carbon. There should be four bonds made by each carbon and one bond by each hydrogen. And bond line and Lewis structures are different.
