Question
Question: Example 2: Hydroboration reaction - conversion of alkynes to aldehydes...
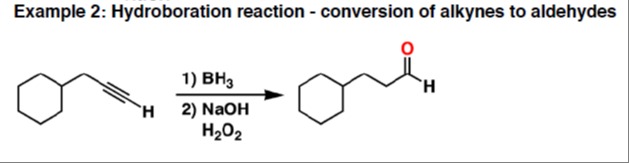
Example 2: Hydroboration reaction - conversion of alkynes to aldehydes
Answer
The reaction of the terminal alkyne with BH₃ followed by NaOH/H₂O₂ yields the aldehyde: cyclohexyl‑butanal (cyclohexyl–CH₂–CH₂–CHO).
Explanation
Solution
-
Step 1: Hydroboration of terminal alkyne
R–C≡CH+\ceBH3⟶vinylborane intermediate
The boron adds to the terminal carbon (anti‑Markovnikov) giving a vinylborane. -
Step 2: Oxidation with alkaline H₂O₂
vinylborane+\ceH2O2/\ceNaOH⟶aldehyde
The boron is replaced by OH, and tautomerisation leads to the aldehyde. -
Overall transformation
Cyclohexyl‑butanal is formed with the carbonyl at the terminal carbon.
