Question
Question: Examine the structural formulas shown below and identify how many compounds will show coupling react...
Examine the structural formulas shown below and identify how many compounds will show coupling reaction with diazonium salts faster than anisole (Ph – Ö – CH3)
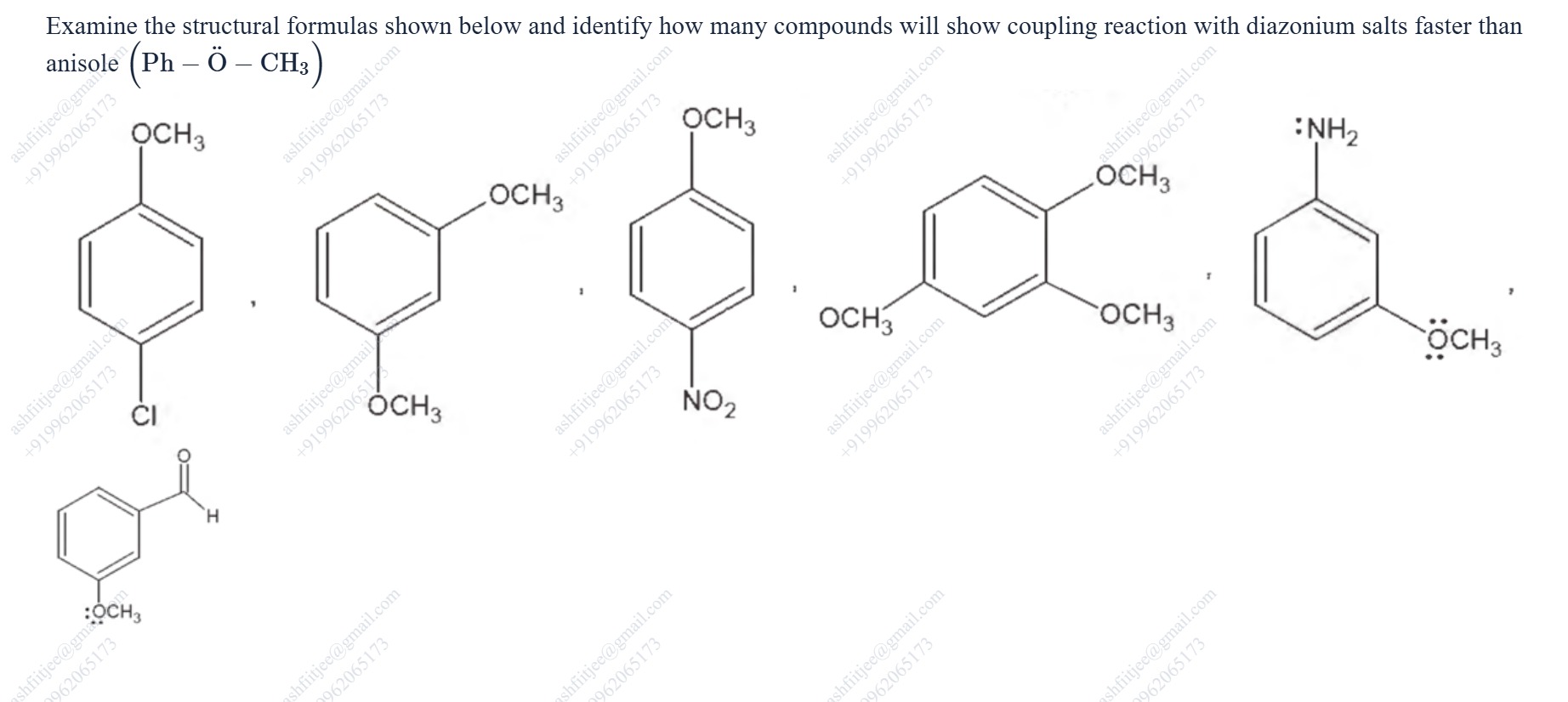
3
Solution
The coupling reaction of diazonium salts with aromatic compounds is an electrophilic aromatic substitution reaction. The rate of this reaction depends on the electron density of the aromatic ring. Electron-donating groups activate the ring towards electrophilic substitution, increasing the reaction rate, while electron-withdrawing groups deactivate the ring, decreasing the reaction rate.
Anisole (Ph-O-CH3) has a methoxy group (-OCH3) which is an activating group due to its strong +R effect (resonance donation of electron density to the ring) and weak -I effect (inductive withdrawal of electron density). The +R effect is dominant, making -OCH3 an overall activating group, directing electrophiles to the ortho and para positions.
We need to identify which of the given compounds are more reactive than anisole towards coupling with diazonium salts. This means we are looking for compounds that are more activated than anisole.
The given compounds are:
- 4-chloroanisole: Contains a methoxy group (activating) and a chlorine atom (weakly deactivating overall, but ortho/para directing). The deactivating inductive effect of chlorine is significant. Compared to anisole, the overall electron density on the ring is likely reduced, making it less reactive.
- 1,3-dimethoxybenzene: Contains two methoxy groups. With two activating groups, the ring is expected to be more activated than anisole with one methoxy group.
- 4-nitroanisole: Contains a methoxy group (activating) and a nitro group (strongly deactivating). The strong electron-withdrawing effect of the nitro group significantly deactivates the ring, making it much less reactive than anisole.
- 1,2,4-trimethoxybenzene: Contains three methoxy groups. With three activating groups, the ring is expected to be significantly more activated than anisole with one methoxy group.
- 3-methoxyaniline: Contains an amino group (-NH2) and a methoxy group (-OCH3). The amino group is a very strong activating group (+R effect). The methoxy group is also an activating group. Aniline is much more reactive than anisole. With an additional activating methoxy group, 3-methoxyaniline is expected to be much more reactive than anisole.
- 3-methoxybenzaldehyde: Contains a methoxy group (activating) and a formyl group (-CHO, moderately deactivating). The deactivating effect of the formyl group is expected to reduce the reactivity compared to anisole.
Comparing the activating effects, we can conclude that compounds with more or stronger activating groups than anisole will be more reactive. Anisole has one -OCH3 group. 1,3-dimethoxybenzene has two -OCH3 groups. 1,2,4-trimethoxybenzene has three -OCH3 groups. 3-methoxyaniline has a -NH2 group (very strong activator) and a -OCH3 group.
Thus, the compounds that will show coupling reaction with diazonium salts faster than anisole are 1,3-dimethoxybenzene, 1,2,4-trimethoxybenzene, and 3-methoxyaniline. There are 3 such compounds.
