Question
Question: Examine the structural formulas of compounds given below and identify number of compounds which show...
Examine the structural formulas of compounds given below and identify number of compounds which show positive iodoform test on vigorous reaction with excess of alkali and I₂
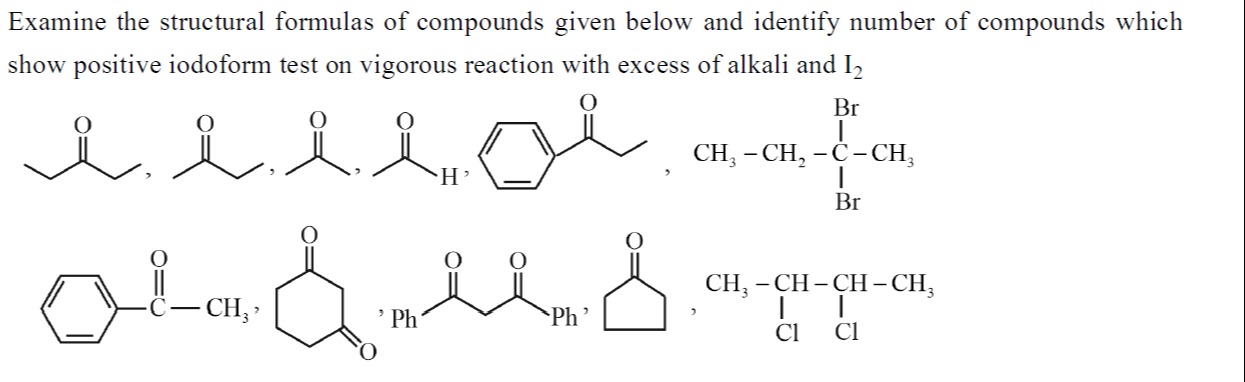
5
Solution
The iodoform test is given by compounds containing a methyl ketone group (R-CO-CH₃) or a methyl carbinol group (R-CH(OH)-CH₃). Under the given conditions of "vigorous reaction with excess of alkali and I₂", compounds that can be converted into these functional groups through hydrolysis or oxidation will also give a positive test.
Let's examine each compound:
-
3-Pentanone (CH₃CH₂COCH₂CH₃): This is a ketone, but it does not have a methyl group directly attached to the carbonyl carbon. It has -CH₂CH₃ groups on both sides. Therefore, it will not give a positive iodoform test.
-
2-Butanone (CH₃COCH₂CH₃): This is a methyl ketone, as it has a -COCH₃ group. Therefore, it will give a positive iodoform test.
-
Acetaldehyde (CH₃CHO): This is a methyl aldehyde, as it has a -COCH₃ group (where R=H). Therefore, it will give a positive iodoform test.
-
Propiophenone (C₆H₅COCH₂CH₃): This is a ketone, but it does not have a methyl group directly attached to the carbonyl carbon. It has a -CH₂CH₃ group. Therefore, it will not give a positive iodoform test.
-
2,2-Dibromobutane (CH₃-CH₂-C(Br)₂-CH₃): This is a geminal dihalide. Under vigorous reaction with excess alkali (hydrolysis), gem-dihalides on the same carbon atom are hydrolyzed to form a ketone.
CH₃-CH₂-C(Br)₂-CH₃ + 2OH⁻ → CH₃-CH₂-CO-CH₃ (2-Butanone) + 2Br⁻ + H₂O
Since 2-Butanone is a methyl ketone, it will give a positive iodoform test. Therefore, 2,2-Dibromobutane will give a positive iodoform test.
-
Acetophenone (C₆H₅COCH₃): This is a methyl ketone, as it has a -COCH₃ group. Therefore, it will give a positive iodoform test.
-
Cyclohexane-1,4-dione: This compound has two carbonyl groups. The carbons adjacent to the carbonyl groups are -CH₂- (methylene) groups. There are no methyl groups adjacent to any carbonyl carbon. Therefore, it will not give a positive iodoform test.
-
1,3-Diphenylpropane-1,3-dione (Dibenzoylmethane) (C₆H₅COCH₂COC₆H₅): This is a β-diketone. The carbons adjacent to the carbonyl groups are a -CH₂- group and phenyl groups. There are no methyl groups directly attached to any carbonyl carbon. Therefore, it will not give a positive iodoform test.
-
Cyclopentanone: The carbons adjacent to the carbonyl group are -CH₂- (methylene) groups. There are no methyl groups adjacent to the carbonyl carbon. Therefore, it will not give a positive iodoform test.
-
2,3-Dichlorobutane (CH₃-CH(Cl)-CH(Cl)-CH₃): This is a vicinal dihalide. Under vigorous reaction with excess alkali (hydrolysis), vicinal dihalides are hydrolyzed to form diols.
CH₃-CH(Cl)-CH(Cl)-CH₃ + 2OH⁻ → CH₃-CH(OH)-CH(OH)-CH₃ (2,3-Butanediol) + 2Cl⁻
2,3-Butanediol contains two methyl carbinol groups (CH₃-CH(OH)- and -CH(OH)-CH₃). These secondary alcohol groups are oxidized by I₂/OH⁻ to form carbonyl groups, leading to butane-2,3-dione (CH₃-CO-CO-CH₃), which contains methyl ketone groups. Therefore, 2,3-Dichlorobutane will give a positive iodoform test.
The compounds that show a positive iodoform test are:
- 2-Butanone
- Acetaldehyde
- 2,2-Dibromobutane
- Acetophenone
- 2,3-Dichlorobutane
Thus, a total of 5 compounds show a positive iodoform test.
