Question
Question: Examine the following structures A.
B.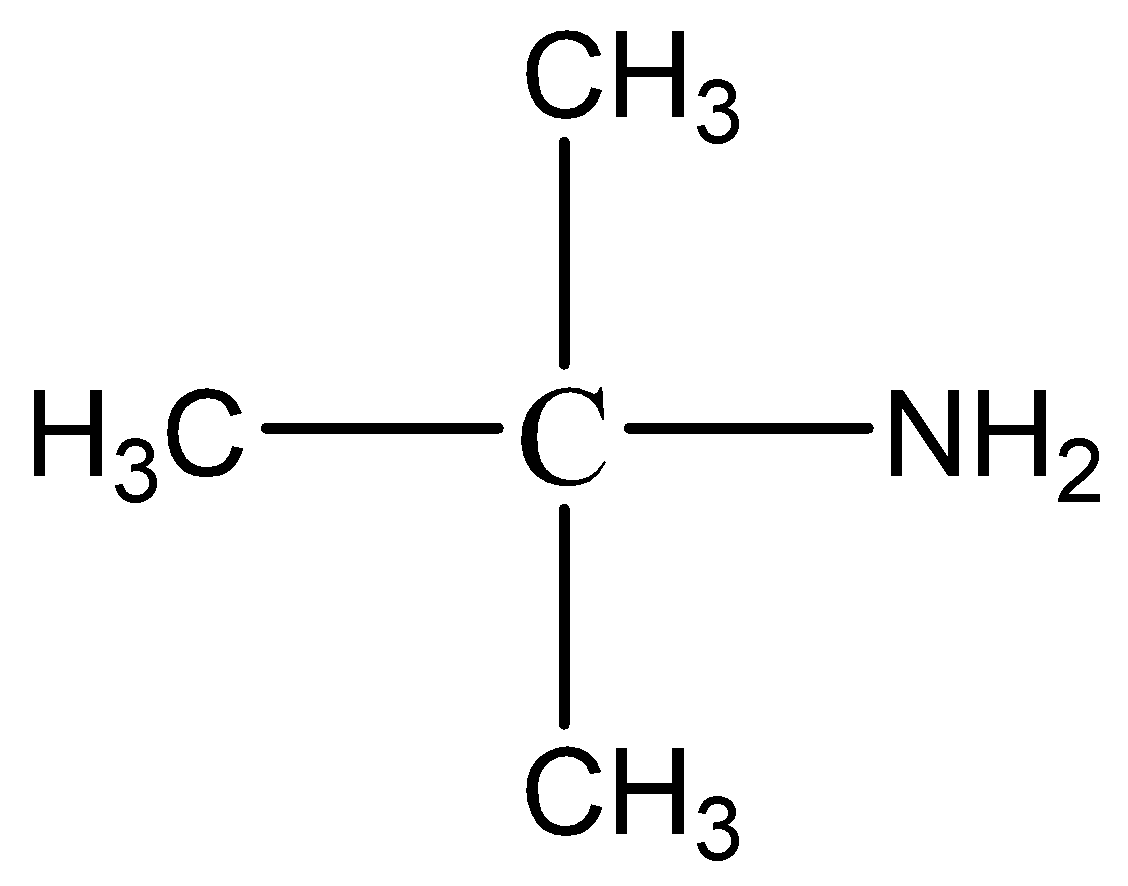
Which of the following statements is correct?
A.A is tertiary alcohol while B is tertiary amine
B.A is primary alcohol while B is primary amine
C.A is tertiary alcohol while B is primary amine
D.A is primary alcohol while B is tertiary amine
Solution
Since tertiary alcohol means OH group is attached to a tertiary carbon and tertiary amine is decided by checking the number of carbons with which N is attached.
Complete answer:
To classify the alcohols as primary, secondary or tertiary alcohols, we look at the number of carbon atoms that are bonded to the carbon atom bearing the OH group and not the Oxygen (O) atom itself. The tertiary carbons are attached to three other carbons. Amines are classified according to the number of carbon atoms bonded directly to the nitrogen atom. A primary (1∘) amine has one alkyl (or aryl) group on the nitrogen atom, a secondary (2∘) amine has two, and a tertiary (3∘) amine has three amino group (NH2) is named as a substituent in more complicated amines, such as those that incorporate other functional groups or in which the alkyl groups cannot be simply named. So, A is tertiary alcohol because the OH group is attached to a tertiary carbon atom (carbon attached to three other carbon atoms) while B is primary amine where nitrogen is attached to one alkyl chain.
Therefore, the correct answer is option (A).
Note: Primary carbons are carbons attached to one other carbon. Secondary carbons are attached to two other carbons. Tertiary carbons are attached to three other carbons. And, quaternary carbons are attached to four other carbons. Primary, secondary, and tertiary amines are nitrogen bound to one, two and three carbons, respectively. Since the nitrogen has a lone pair, it is still possible to form another bond to carbon. These are called quaternary amines, although they bear a positive charge on nitrogen and are not at all basic and they are often referred to as quaternary ammonium salts.
