Question
Question: Ethylene reacts with \(B{r_2}\) to give 1, 2 dibromoethane. The anti-addition takes place due to the...
Ethylene reacts with Br2 to give 1, 2 dibromoethane. The anti-addition takes place due to the formation of the intermediate__________.
A. CH2Br−CH2
B.
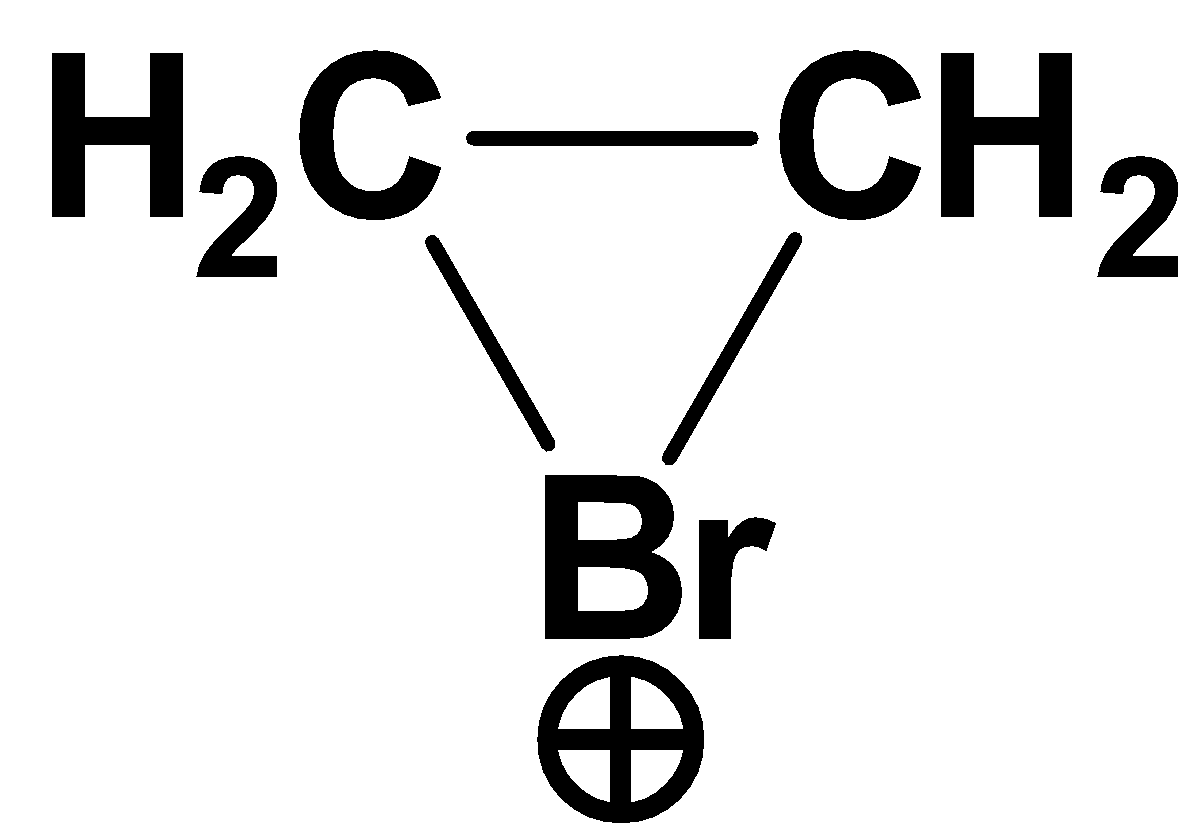
C. BrCH=CH+
D.
Solution
Carbon is a tetravalent atom with four valence electrons which means it has 4 electrons with which it can bond to up to for atoms. Carbon is also able to form multiple bonds like double or triple bonds and thus form a wide range of compounds. The strength of the triple bonds is higher than that of double bonds and is thus difficult to break.
Complete step by step answer:
Alkenes and alkynes react in the cold water and bromine to form a dibromine substituted product. The reaction takes place in the presence of an organic solvent like the tetrachloromethane.
During the process, the multiple bonds of the compound break, and bromine atoms get attached to the carbon atoms.
When it comes to double bonds stereochemistry also comes to play. The addition of bromine atoms to the compound could be either syn or anti. The bromine atoms may attach to the compound to form the compound which is trans in nature or cis in nature.
This may lead to cis-trans isomerism. The external conditions and the intermediate which are formed decide the formation of the product. The reagent used in the reaction also helps in deciding which product is formed.
The formation of the compound takes place through the formation of intermediates. This intermediate helps in the movement of the reaction in the forward direction.
Ethylene reacts with to give 1,2 dibromomethane. The bromine added is added in an anti-manner. The anti-addition takes place due to the formation of the cyclic bromonium ion as an intermediate.
The bromonium ion can be represented as
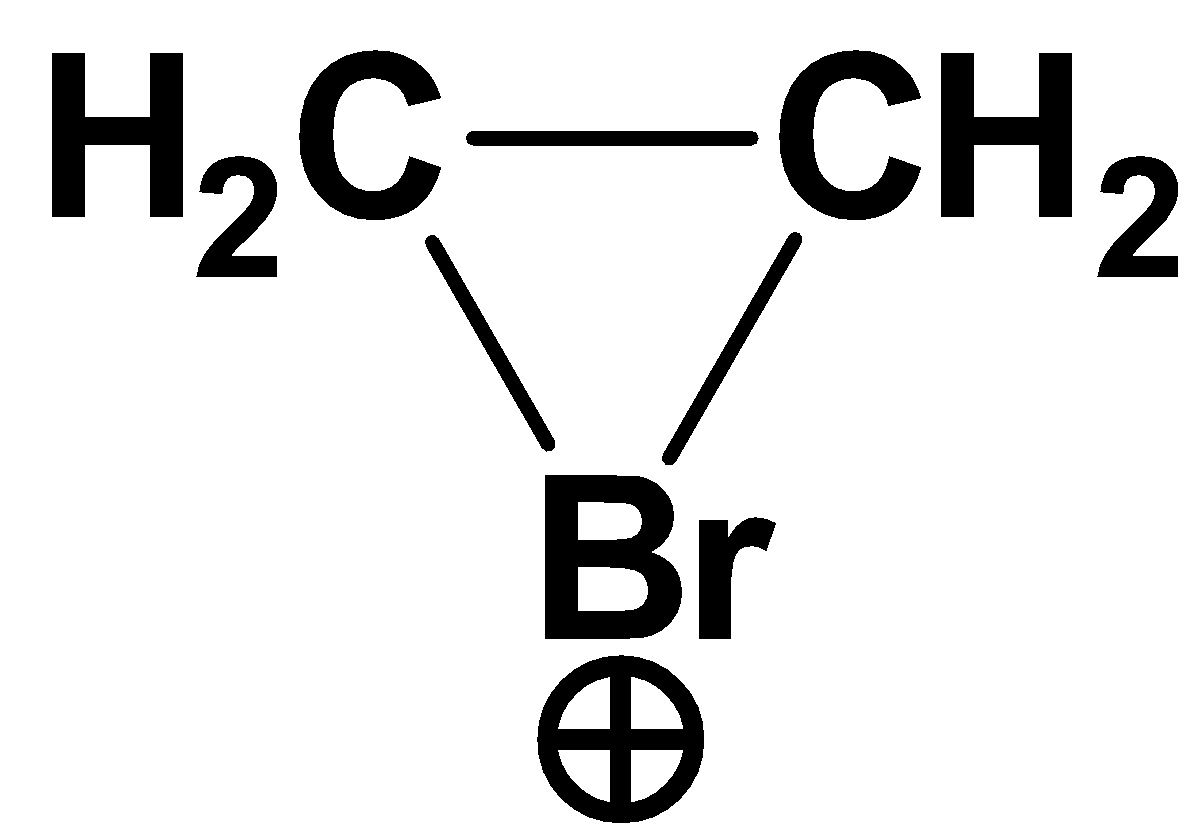
So, the correct answer is Option B.
Note: The process shown is called bromination. The addition of a halogen to the compound is called halogenation since the halogen added here is bromine hence called bromination.
The halogens comprise the elements in group 17 of the periodic table. They are usually highly electronegative and have a big size.
