Question
Question: Ethylene on the addition of hypochlorous acid forms: (A)  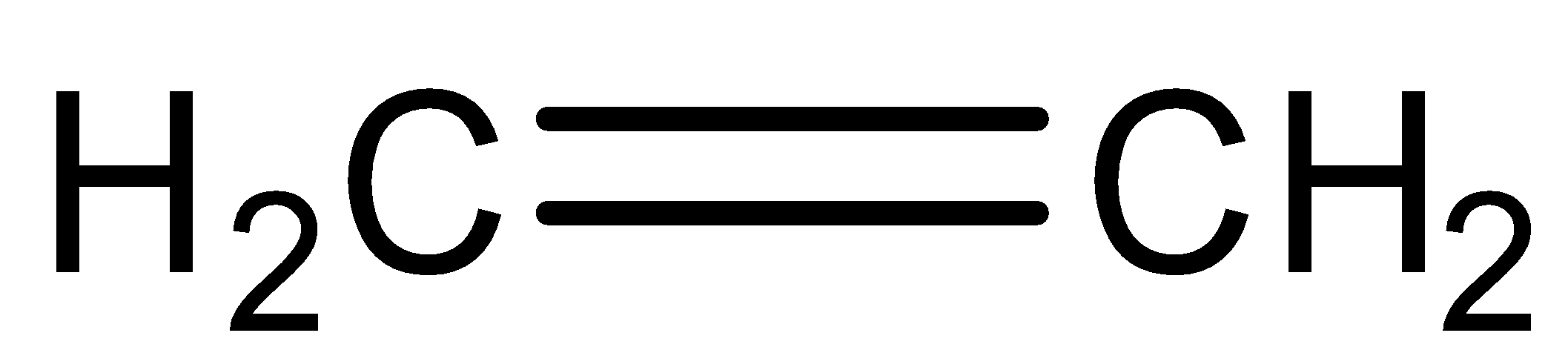
(B) 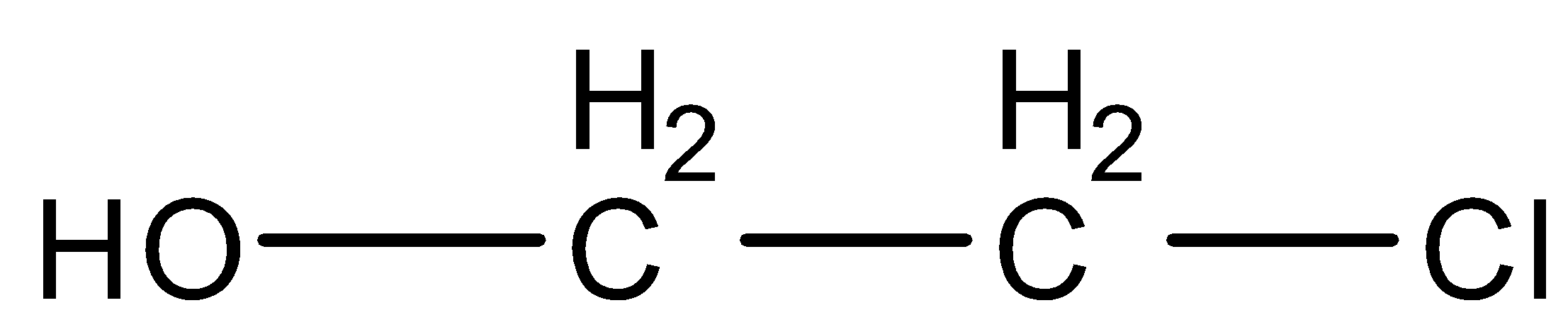
(C) 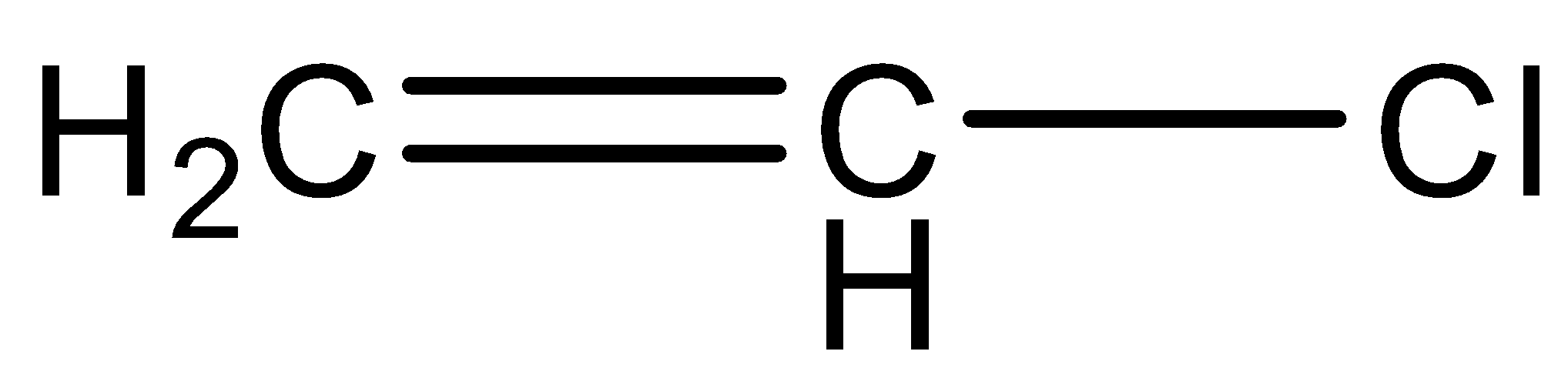
(D) 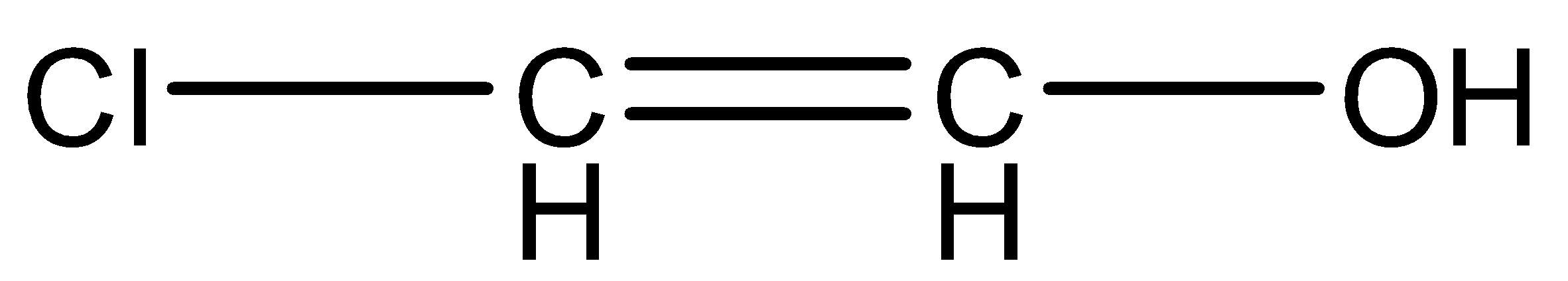
Solution
The reaction between ethylene 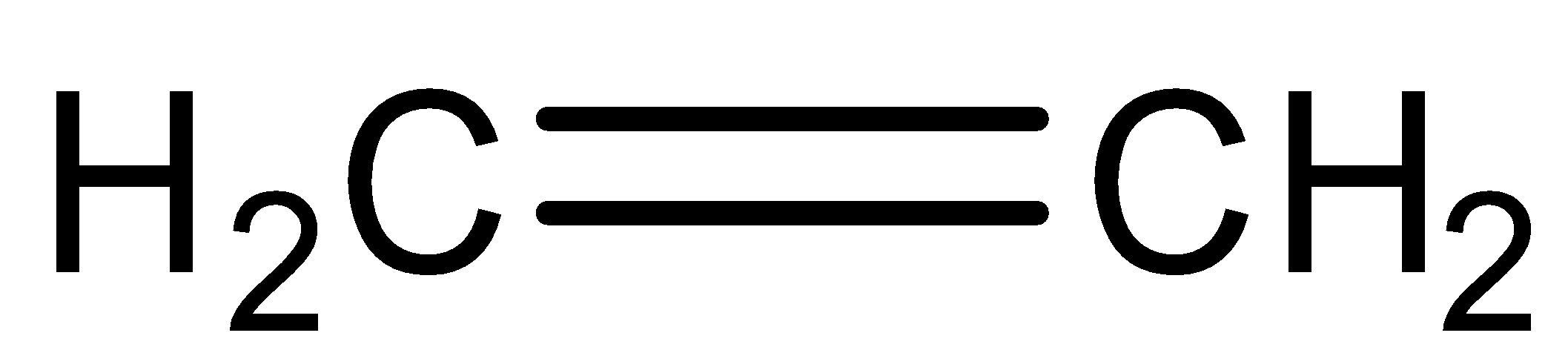 on the addition of hypochlorous acid HOCl depends on the amount of HOCl added as well as Markovnikov’s rule. Also, there is breaking as well as the formation of bonds
on the addition of hypochlorous acid HOCl depends on the amount of HOCl added as well as Markovnikov’s rule. Also, there is breaking as well as the formation of bonds
Complete step by step solution:
First, we will understand the basic principle used for the reaction that is Markovnikov’s rule. According to Markovnikov’s rule, the electrophilic addition reaction of alkenes and alkynes have proceeded with the addition of halogen atoms to the carbon atom bearing the maximum number of hydrogen atoms. Now we will write the chemical reaction which shows the additional reaction of ethylene and hypochlorous acid. The reaction is given below:

Now we will discuss the reaction step by step. In the first step of the reaction, the double bond of ethylene and the single bond of hypochlorous acid breaks which results in the formation of electrophile and nucleophile. Therefore, there is a negative charge over the hydroxyl group OH− and the positive charge is formed over the halogen group Cl+ , which results in the formation of +CH2−CH2−Cl . Now we will discuss the second step of the reaction. In this step, OH− gets attached with +CH2−CH2−Cl and forms the final product OH−CH2−CH2−Cl . The final product formed is Ethylene chlorohydrin. The IUPAC name of the product formed is 2−chloro−ethanol .
Therefore, Ethylene on the addition of hypochlorous acid forms 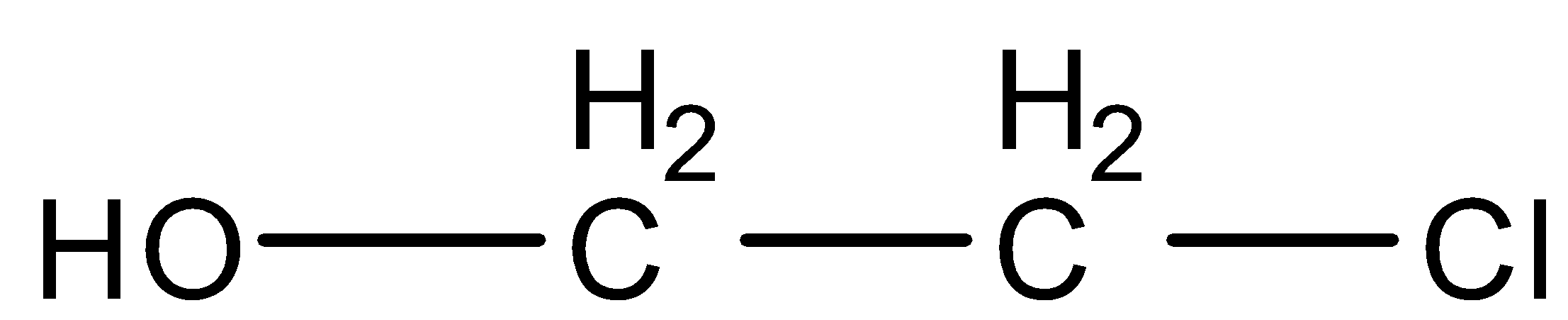 .
.
Therefore, the correct option is (B).
Note:
The product formed is Ethylene chlorohydrin is an organochlorine compound and hazardous substance. It is used as a solvent and in the manufacture of a variety of industrial agents. It is also used as a xenobiotic metabolite.
