Question
Question: Ethyl formate ester reacts with \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}...
Ethyl formate ester reacts with CH3CH2CH2MgBr to give secondary alcohol. The alcohol formed is
A) 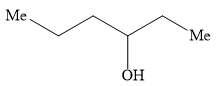
B) 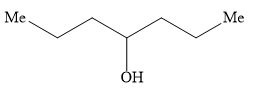
C) 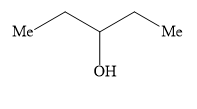
D) 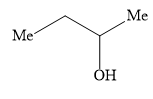
Solution
Formic acid contains one carbon atom. Propyl magnesium bromide contains three carbon atoms. The product will have seven carbon atoms.
Complete answer:
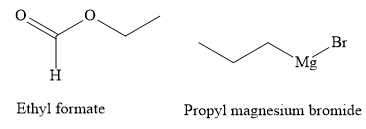
The structures of the starting materials ethyl formate and propyl magnesium bromide are as shown below:
Write the chemical reaction
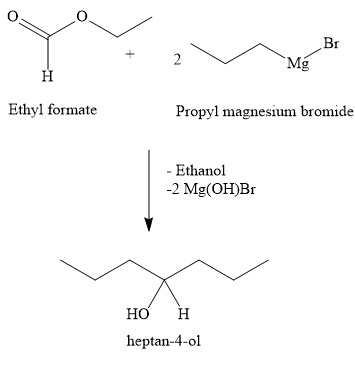
Two moles of propyl magnesium bromide react with one mole of ethyl formate to form heptan-4-ol. Propyl magnesium bromide is a Grignard reagent. Ethyl formate is an ester. Heptan-2-ol is a secondary alcohol. Thus, one mole of ester reacts with two moles of grignard reagent to form one mole of heptan-2-ol. In the reaction, two moles of Mg(OH)Br and one mole of ethanol are the side product.
Hence, the option B ) heptan-4-ol is the correct option.
Additional Information: An ester of formic acid reacts with two equivalents of a grignard reagent to form a secondary alcohol. An ester reacts with two equivalents of a grignard reagent to form a tertiary alcohol. An aldehyde reacts with one equivalent of grignard reagent to form secondary alcohol. A ketone reacts with one equivalent of grignard reagent to form tertiary alcohol.
Note: If only one equivalent of propyl magnesium bromide reacts, then ethyl formate will give butane aldehyde. Since in the question, it is mentioned that the product is a secondary alcohol, two equivalents of propyl magnesium bromide will react with ethyl formate to form heptan-4-ol. In the secondary alcohol, the carbon atom bearing hydroxyl group has one hydrogen atom and two other carbon atoms.
