Question
Question: Ethyl ether when reacted with chlorine in dark yields: A. \(C{{H}_{3}}CHClOC{{H}_{2}}C{{H}_{3}}\) ...
Ethyl ether when reacted with chlorine in dark yields:
A. CH3CHClOCH2CH3
B. CH3CH2OCH2CH2Cl
C. ClCH2CH2OCH2CH2Cl
D. CH3CHClOCHClCH3
Solution
To solve this question firstly we have to draw the structure of ethyl ether and then we have to use the concept of halogenations of ether. Halogenation of ether is a reaction where ether reacts with halogens. Here when ethyl ether tends to react with chlorine in dark then at the alpha carbon atom substitution occurs.
Complete answer:
From your chemistry lesson you have learned about the halogenations of et5her and the product that is formed when ether reacts with halogen in the dark.
Halogenation of ether is a reaction where ether reacts with halogens. Here when ether tends to react with chlorine in dark then at the alpha carbon atom substitution occurs. Dark reaction is that chemical reaction which does not require light for that reaction.
Now to solve this question we have to draw the structure of ethyl ether and as we know that ether is a type of compound where two aryl or alkyl or one aryl and one alkyl is bonded with the oxygen. Ether can also be formed through the dehydration of alcohols by emitting the water molecules with the use of dehydration agents.
So, the structure of ethyl ether will be:
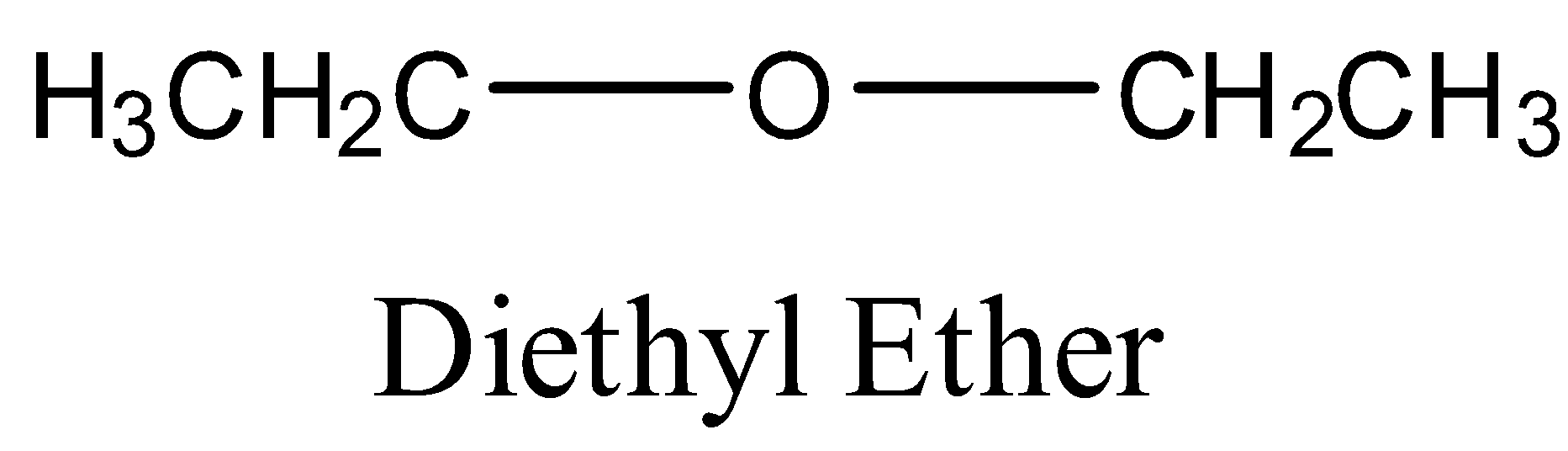
Now, in the question we are asked to find the product when ethyl ether reacts with chlorine (Cl2) . So, when ethyl ether will react with chlorine in dark then substitution will occur at the α- carbon atoms. α-carbon atoms refer to the first carbon atom which attaches to a functional group. Therefore the yield of the reaction will be α-chloro diethyl ether or 1-chloro diethyl ether.
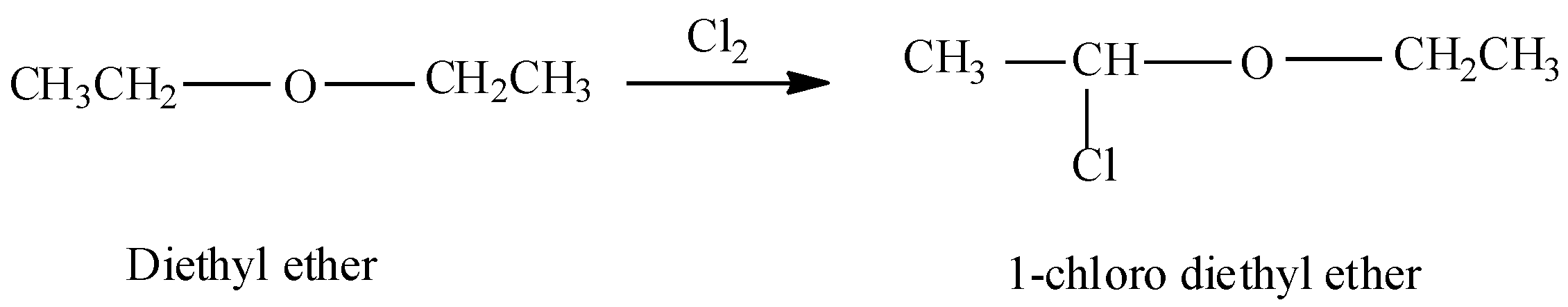
Therefore ethyl ether when reacted with chlorine in dark yields CH3CHClOCH2CH3.
Thus the correct option will be (A).
Note:
Ethers can undergo combustion reactions where they react with oxygen to produce carbon dioxide and water. Ethers are highly flammable and thus react with halogen to form halogen-substituted ether which undergoes substitution reaction in absence of light or in dark. It is an excellent solvent for the dyes and it is also used in the laboratories.
