Question
Question: Etherates are: A. Ethers B. Solution in ether C. Complex of ethers with Lewis acid D. Comple...
Etherates are:
A. Ethers
B. Solution in ether
C. Complex of ethers with Lewis acid
D. Complex of ether with Lewis base
Solution
Etherates are the compounds of an ether that includes especially diethyl ether and the other chemical compounds i.e., boron trifluoride etherate.
Complete answer:
Etherates are the compounds that form complexes between an ether and the Lewis acid. An example of etherate is boron trifluoride diethyl etherate with a chemical formula BF3O(C2H5)2 and it is a colourless chemical liquid. It is used as the source of the boron trifluoride in many of the chemical reactions which requires a Lewis acid. BF3 acts a Lewis acid that accepts an electron pair and has vacant orbitals.
The reaction involve via equilibrium is given below:
BF3O(C2H5)2⇌BF3+O(C2H5)2
BF3 acts as a Lewis acid and it even binds to the weak Lewis base that includes the reactions of resulting adducts with the nucleophile.
Thus, the correct answer is option is C. i.e., Complex of ethers with Lewis acid.
Additional information:
Lewis acids are the species that accept an electron pair (electrophile) and they have vacant orbitals. While the species that donates an electron pair (nucleophile) and they have lone pair of electrons are known as Lewis base.
Note:
The structure of boron trifluoride diethyl etherate is given as:
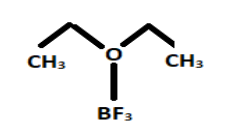
It is a colourless chemical compound that sometimes seems to be brown in the colour. Etherates are the chemical compounds that form complexes with Lewis acid in which boron trifluoride acts as a Lewis acid i.e., an electrophile.
