Question
Question: Ethanoic propanoic anhydride on reaction with excess MeMgBr gives the major product. (a)- - 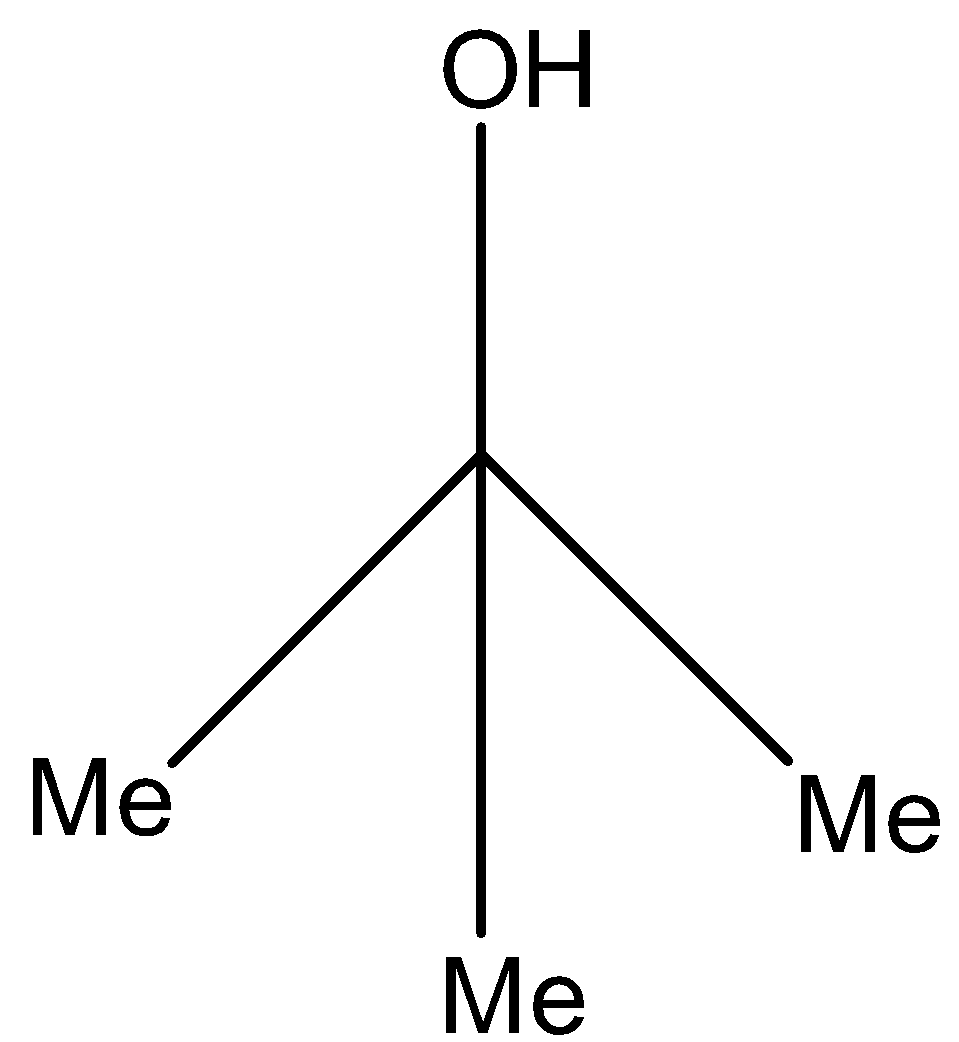
(b)- 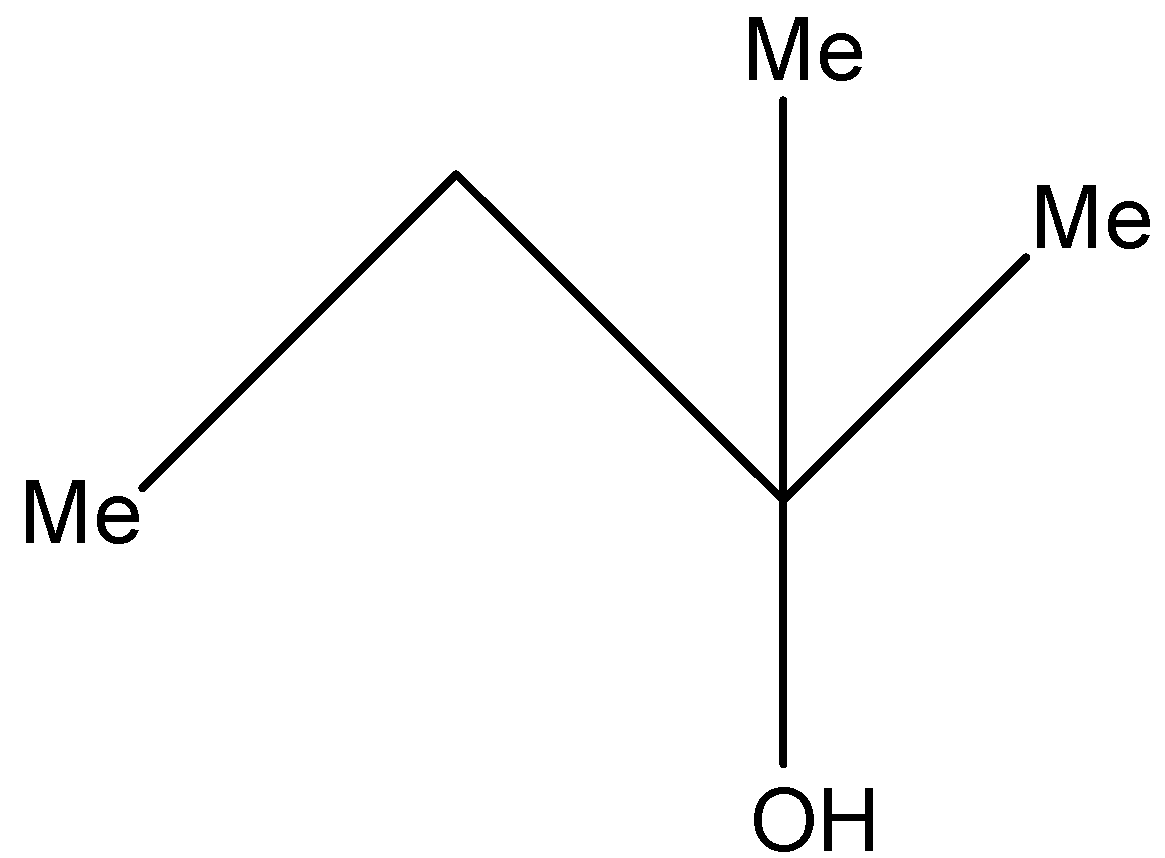
(c)- 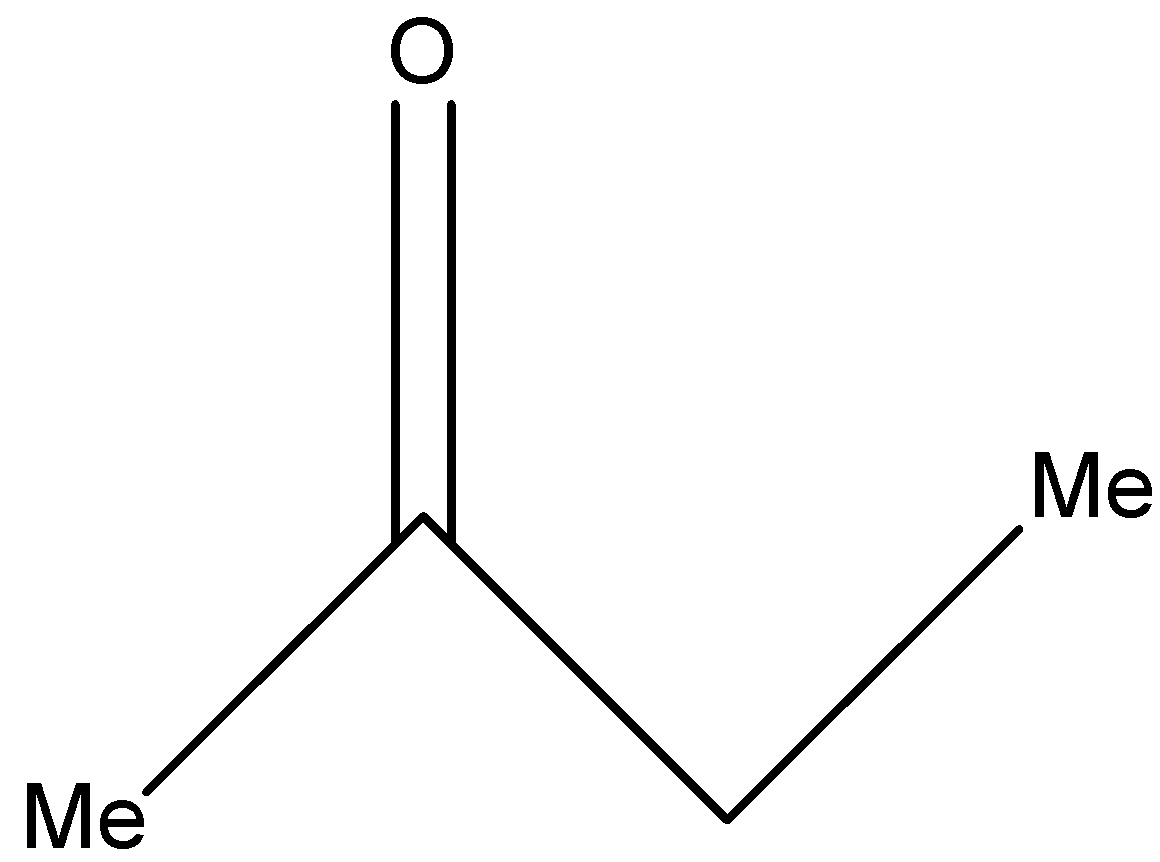
(d)- 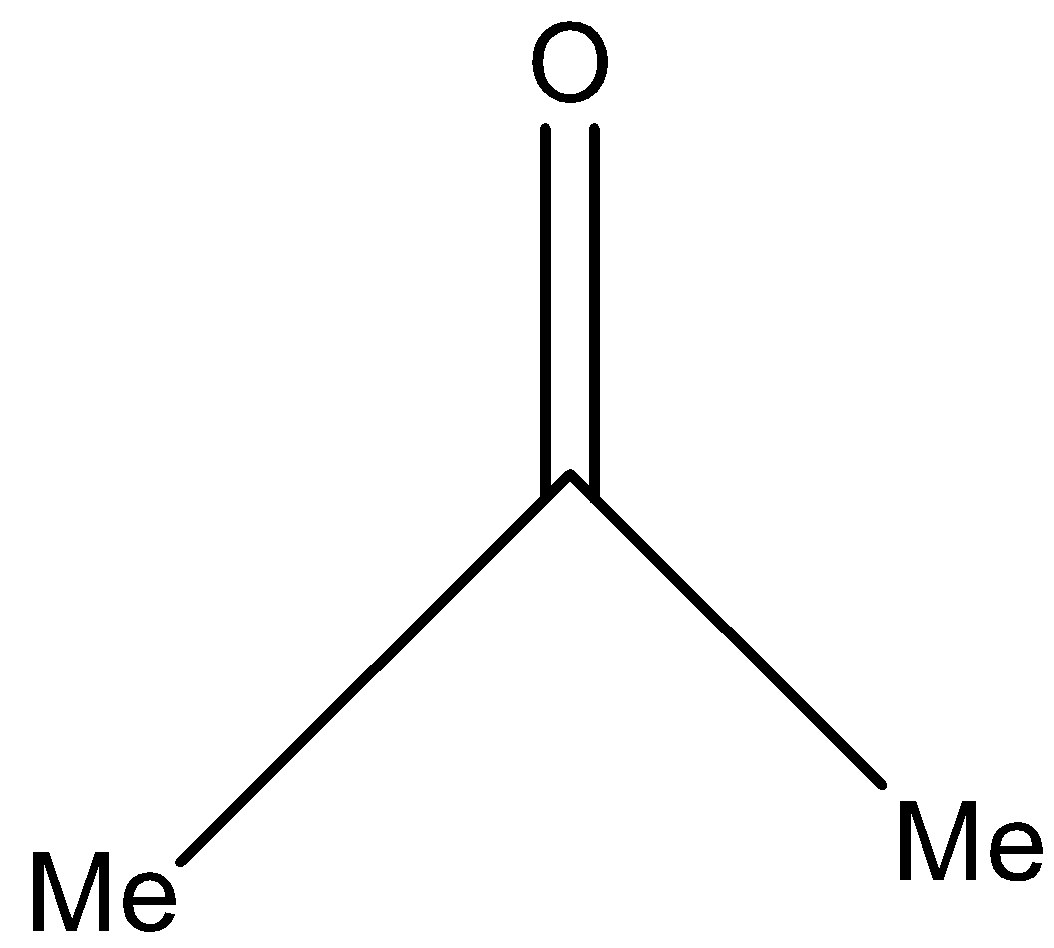
Solution
The formula of ethanoic propanoic anhydride will be CH3−CO−O−CO−CH2CH3, and this compound reacts with an excess of MeMgBr means two times there will be a reaction in which at each step there will be the addition of methyl group.
Complete answer:
The ethanoic propanoic anhydride contains a total of five carbon atoms in which the central part is an anhydride. One side of the anhydride is ethanoic and the other part of the anhydride is propanoic. So, the formula of ethanoic propanoic anhydride will be CH3−CO−O−CO−CH2CH3, and the structure will be:
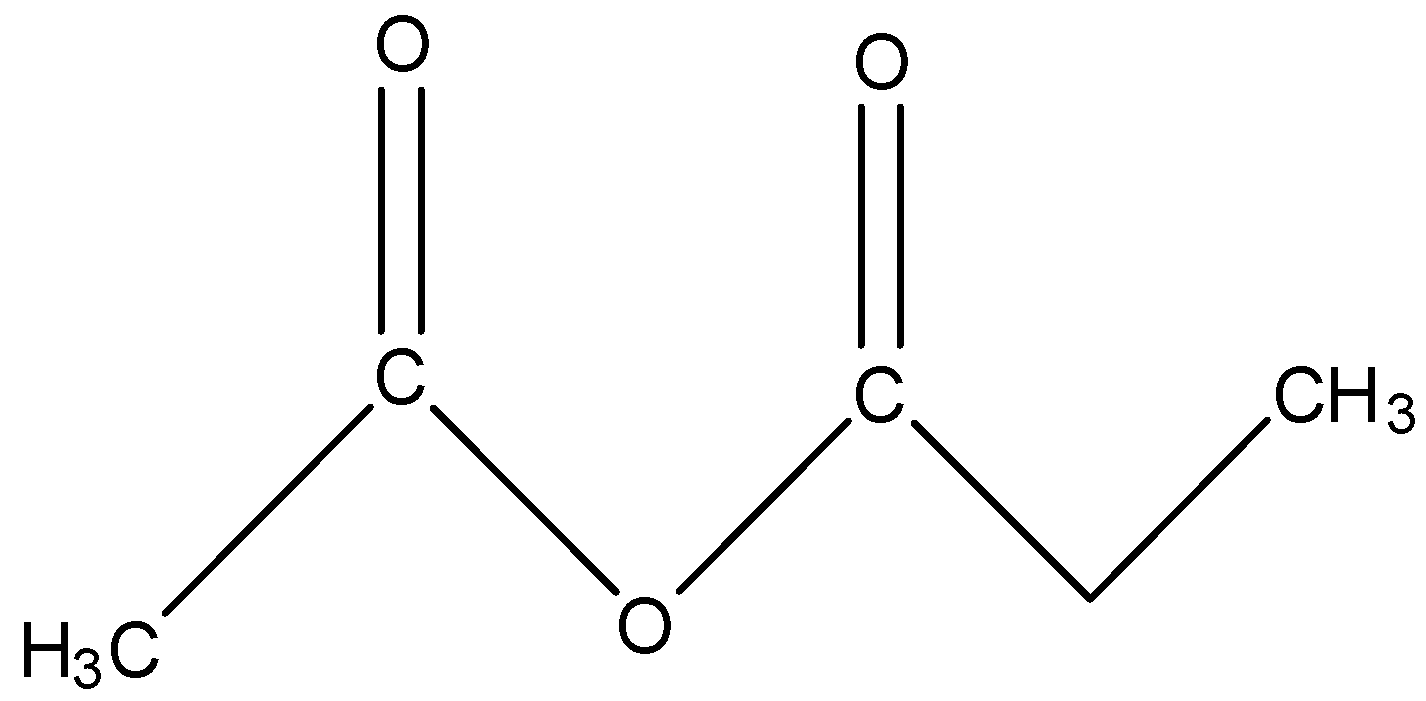
When this compound reacts with MeMgBr then the C-O bond will break and there will be the addition of methyl group. The methyl group will attack because the negative part in MeMgBr will be Me and the MgBr will be the positive part. The reaction is given below:
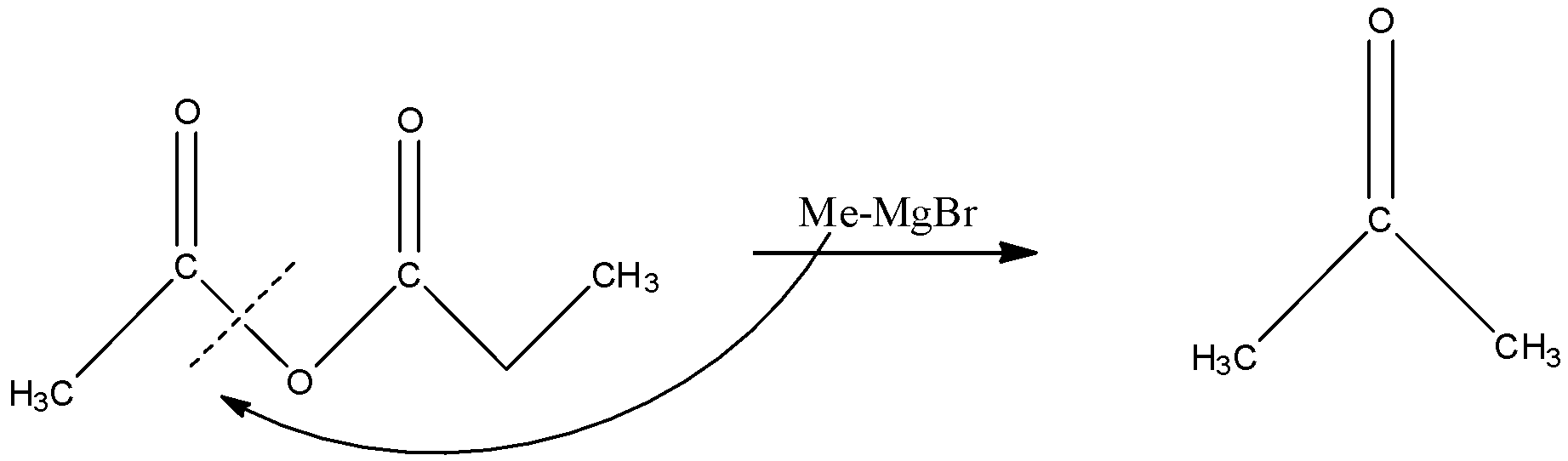
Now we are given that the MeMgBr is in excess quantity which means that the MeMgBr will react once again. In the next step, the methyl group will react with the C=O bond of the acetone. This will create a negative charge on the oxygen atom and a positive charge on the carbon atom, and the methyl group will attack the carbon atom. The reaction is given below:
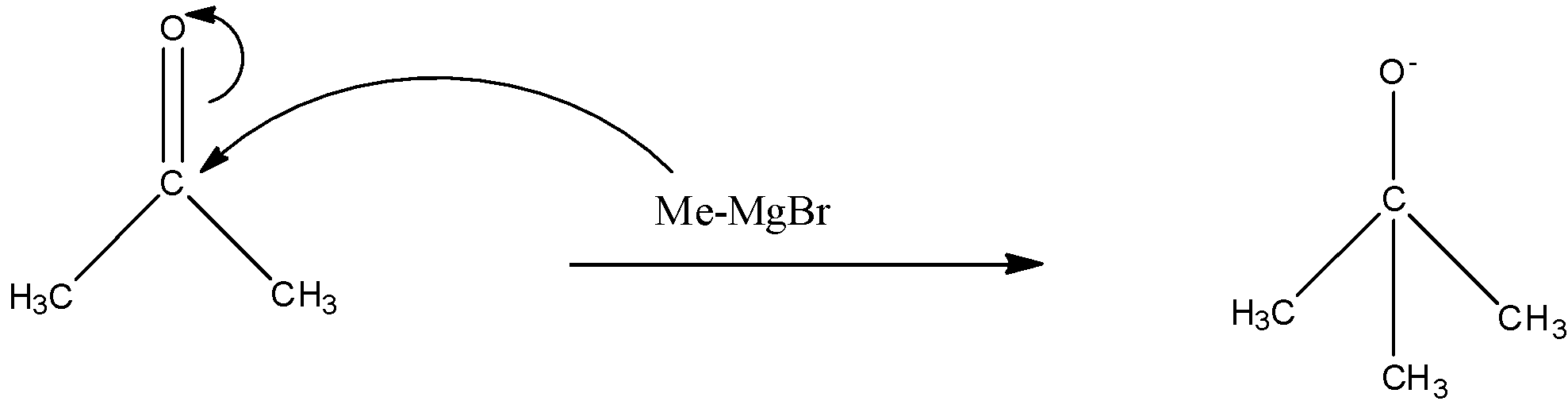
Now on this compound, the hydrogen ion will attack and will form 2-methyl-propan-2-ol. The reaction is given below:
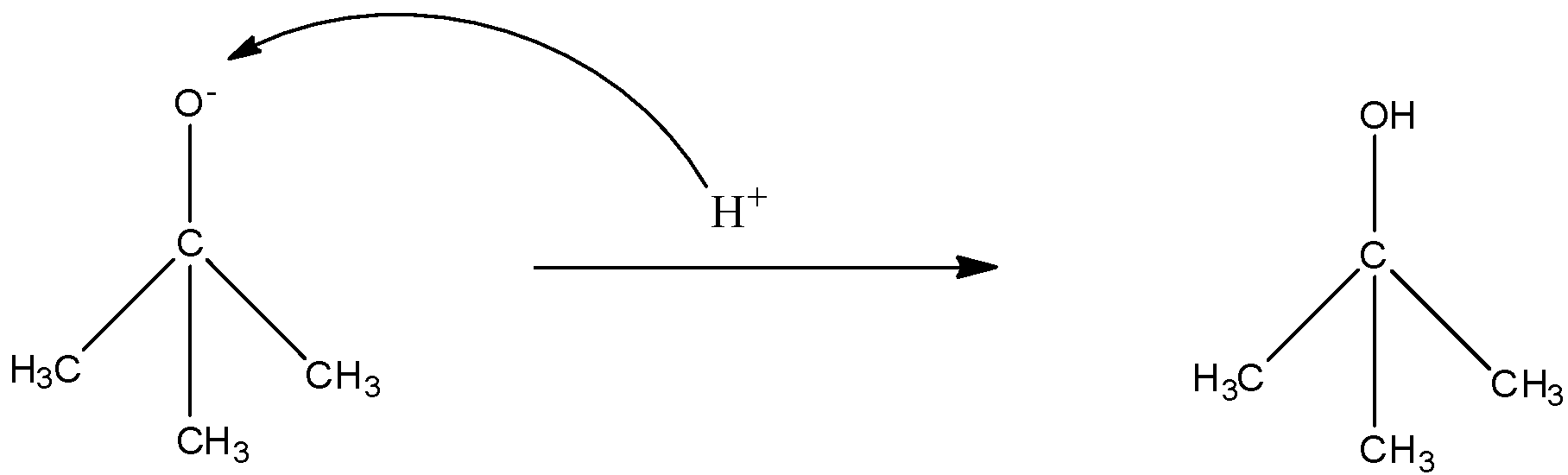
Therefore, the correct answer is an option (a).
Note:
It must be noted that the C-O bond in the ethanoic propanoic anhydride will break from the less substituted side because the Nucleophilic addition will take place at the less substituted part.
