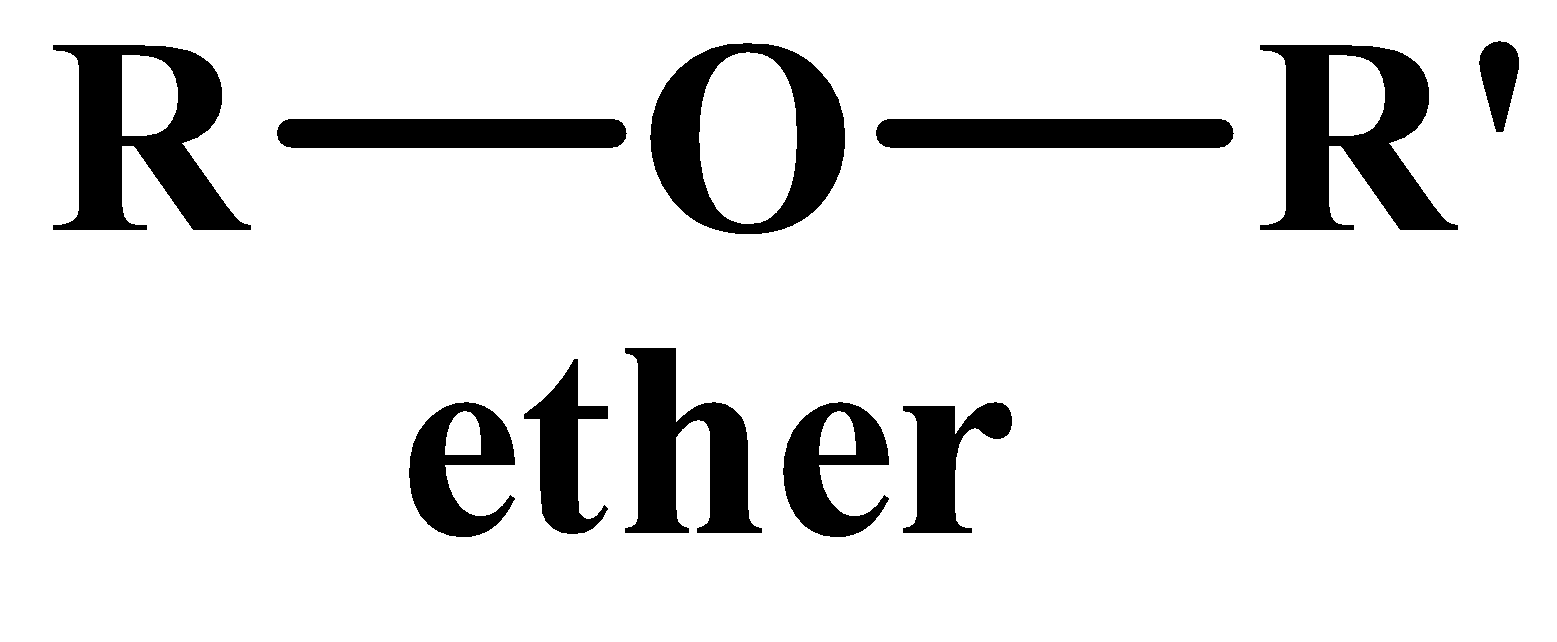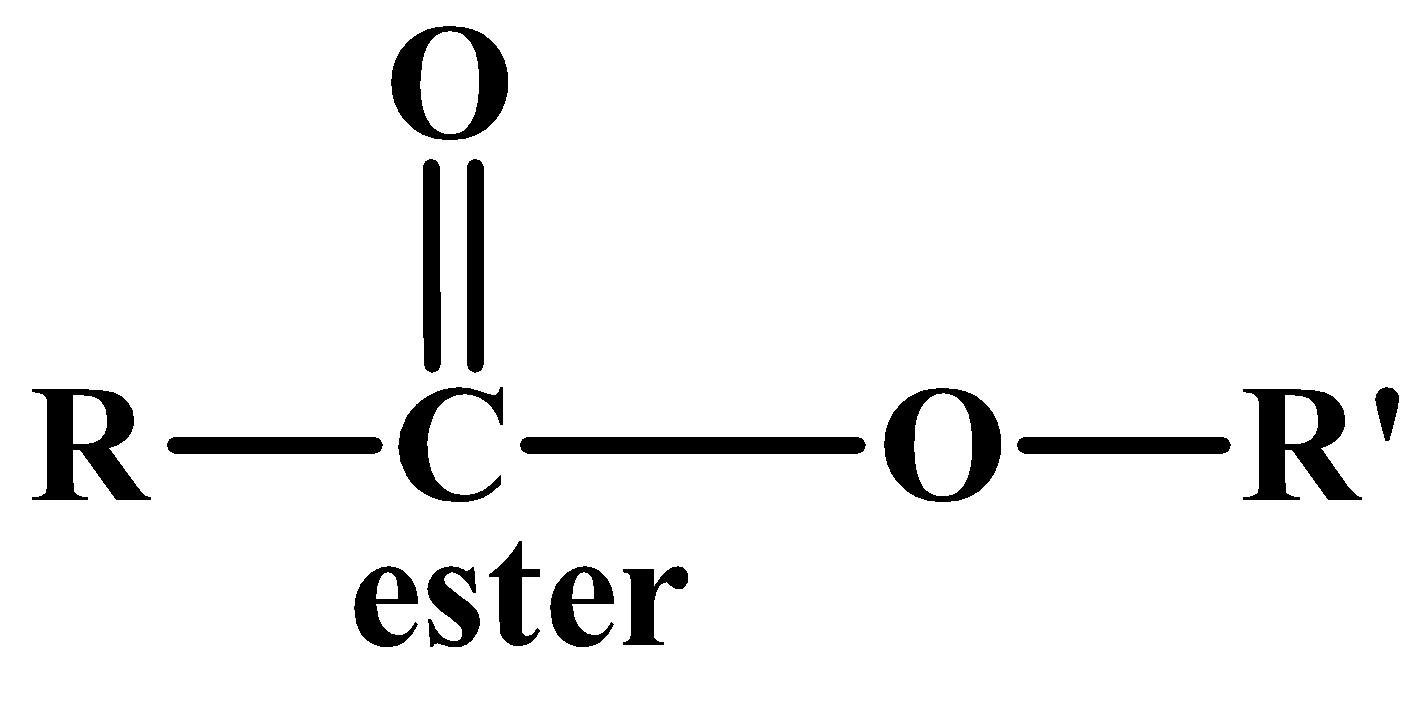Question
Question: Ethanoic acid is a member of Homologous series with general formula \({{C}_{n}}{{H}_{2n+1}}COOH\). ...
Ethanoic acid is a member of Homologous series with general formula CnH2n+1COOH.
(i) Name the series and give its functional group.
(ii) Give the molecular formula and the common name of ethanoic acid.
(iii) If this compound is mixed with ethanol in the presence of Conc.H2SO4, a sweet smelling compound is formed. Give the equation and name the compound.
(iv) Ethanoic acid reacts with carbonates. Which gas is liberated during this reaction?
(v) Write the balanced equation for the reaction of ethanoic acid with carbonate.
(vi) Your grandmother has prepared mango pickle. What has she added to preserve it for a long time?
Solution
Hint:
(i) The functional group is related to carboxylic acid.
(ii) It is commonly used in our homes for preparing food items. It has a sour taste.
(iii) This compound has a fruity smell. The reaction takes place only in an acidic or basic medium.
(iv) The gas is a greenhouse gas. It is exhaled by all organisms undergoing respiration.
(v) Take any metal carbonate. Arrange all the products and then try to balance it.
(vi) Chemically it can be many different products ranging from acids, to salts, nitrites etc. Naturally many different substances are added to make them.
Complete step by step solution:
(i) Carboxylic acids are a homologous series in which the compounds contain a functional group called the carboxyl group (−COOH ). The general molecular formula for carboxylic acids is CnH2n+1COOH.
Carboxylic acids contain at least one carboxyl group. With two or more carboxyl groups attached, they are called dicarboxylic acids, tricarboxylic acids, etc.
They are derivatives of hydrocarbons in which one or more of the hydrogen atoms have been replaced by a carboxyl group.
(ii) Ethanoic acid, popularly known as acetic acid, is a colourless liquid organic compound with the chemical formula CH3COOH. Vinegar is a common cooking ingredient and is composed of acetic acid as its main component.
(iii) Ethanoic acid reacts with ethanol in the presence of concentrated sulphuric acid as a catalyst to produce the ester-ethyl ethanoate. The reaction is slow and reversible. To reduce the chances of the reverse reaction happening, the ester is distilled off as soon as it is formed. This is a direct application of Le Chatelier’s principle.
CH3COOH+C2H5OHCH3COOC2H5+H2O
The above reaction can also happen using base as a catalyst.
(iv) Ethanoic acid reacts with carbonates and hydrogen carbonates to give salt, water, and carbon dioxide. Therefore, the gas released is carbon dioxide.
(v) The reaction equation is as follows:2 CH3COOH + Na2CO3 → 2 CH3COONa + H2O + CO2↑
(vi) Your grandmother can add vinegar in her mango pickle bottle to preserve the pickle for a longer period.
Apart from that, pickles soaked in salt brine can also avoid bacteria and allow good fermentation. This will increase the taste of the pickle and add good flavour. To preserve large quantity pickles, people can use some preservatives such as sodium benzoate. Often fermentation is used to preserve certain food items such as cheese.
Note:
Often esters and ethers are mixed together and many students face problems distinguishing between them. Here are two points to keep in mind:
- esters are formed from the reaction between acids and alcohols whereas ether are formed only from alcohols.
- The general formula of esters has a carbonyl group whereas ethers only have an oxygen atom that covalently links two groups.