Question
Question: Ethane, with the molecular formula \({C_2}{H_6}\) has A.6-covalent bonds B.7-covalent bonds C....
Ethane, with the molecular formula C2H6 has
A.6-covalent bonds
B.7-covalent bonds
C.8-covalent bonds
D.9-covalent bonds
Solution
We can say a covalent bond is a chemical bond formed by combination of two nonmetals (or) by combination of a nonmetal with a metalloid through the sharing of electron pairs between atoms.
Complete step by step answer:
We can define covalent bond as a bond that is formed by sharing of electrons between two atoms (or) two (or) more atoms.
For example: Hydrogen chloride contains covalent bonds
The type of bonding in hydrogen chloride is covalent. The covalent bond is formed between nonmetallic hydrogen and nonmetal chlorine. The mutual sharing of electrons takes place between nonmetallic hydrogen and nonmetallic chlorine.
We have to know that carbon has valency of four. As carbon needs to be stable, (or) to reach the stability it combines with other atoms through electron sharing and forms covalent bonds.
As per the given question, we have found the number of covalent bonds in ethane.
We know the molecular formula of ethane is C2H6. Let us now draw the structure of ethane,
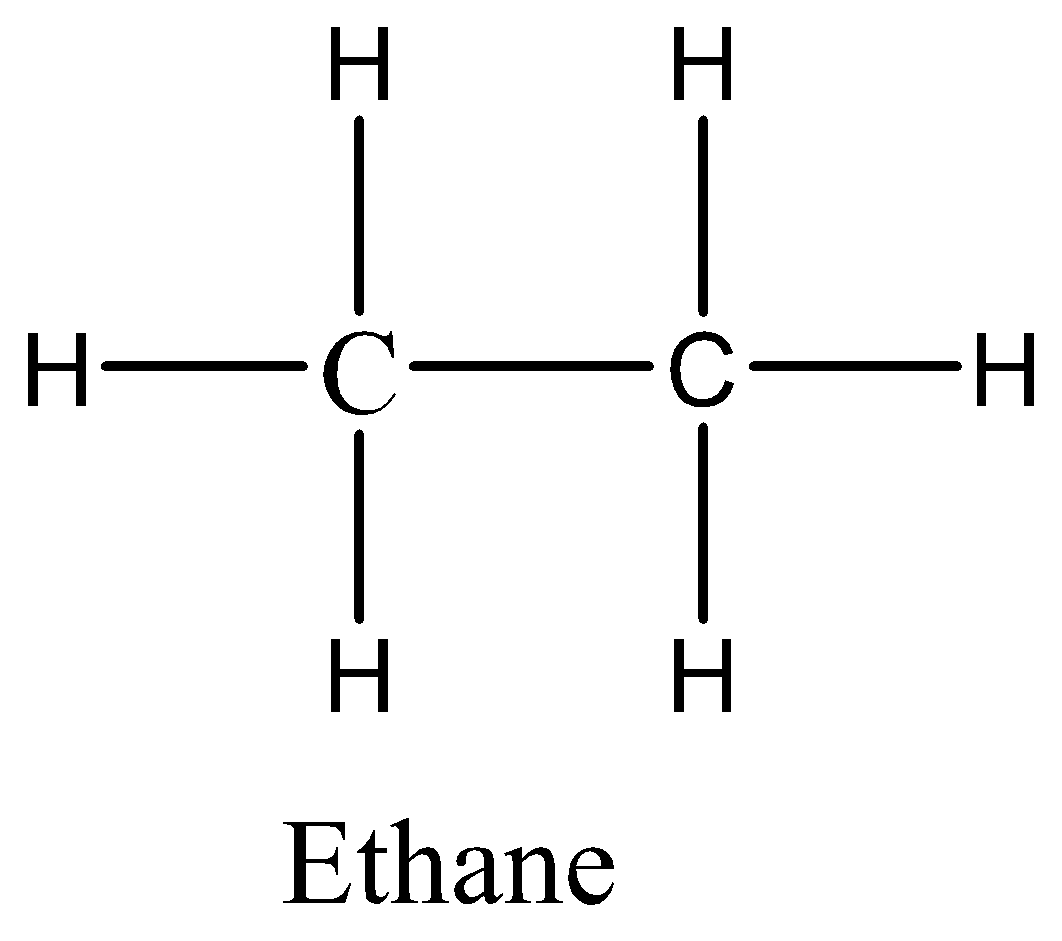
In the structure of ethane, we can see that each carbon atom is bonded to three hydrogen atoms and each carbon atom is bonded to another carbon atom. Six bonds are formed between carbon and hydrogen, one bond is formed between carbon and carbon. The six atoms of hydrogen would share their electrons with carbon to get six carbon-hydrogen covalent bonds. The two atoms of carbon would share their electrons to get carbon-carbon covalent bonds. So, there are totally seven covalent bonds.
So, the correct answer is Option B.
Note:
Now we can discuss about the some of the inorganic compounds that forms covalent bonds are,
1.Sulfur dioxide/sulfur trioxide is formed by the combination of sulfur (a non-metal) and oxygen (a nonmetal). The type of bond between sulphur and oxygen is covalent as the bonds are formed by mutual sharing of electrons between the electrons of sulfur and oxygen.
2.Bromine is a nonmetal and it forms covalent bonds. Two bromine atoms can be joined by a covalent bond because the shared pair of electrons is attracted to the nucleus of both bromine atoms.
3.In carbon dioxide, the type of bond formed between carbon and oxygen is covalent because the electrons are mutually shared between the atoms of carbon and oxygen. Carbon and oxygen are nonmetals.
Some of the uses of ethane are,
1.Used in production of ethene.
2.In cryogenic refrigeration system, ethane is used as refrigerant.
