Question
Question: Ethane with the molecular formula \({{C}_{2}}{{H}_{6}}\) has: A. 6 covalent bonds B. 7 covalent ...
Ethane with the molecular formula C2H6 has:
A. 6 covalent bonds
B. 7 covalent bonds
C. 8 covalent bonds
D. 9 covalent bonds
Solution
Predict the structure of ethane based on the IUPAC name that is given and then count the number of covalent bonds present in the molecule. The bonds can be C−H or C−C.
Complete step by step answer:
First, we will check what type of organic molecule the given molecule is based on its IUPAC name. The given name is ‘ethane’, we can divide this name into two parts, the prefix ‘eth-’ and the suffix ‘-ane’. Here, the prefix indicates that the given compound has 2 carbon atoms which have been given in the molecular formula. The suffix ‘-ane’ indicates that this is an alkane, which means that it does not have any double bonds present between the carbon atoms. We can deduce this from the molecular formula itself is you are familiar with the general formula for alkanes i.e. CnH(2n+2). The given formula also fits into this structure.
Now, let us see the structure of ethane. It is as follows:
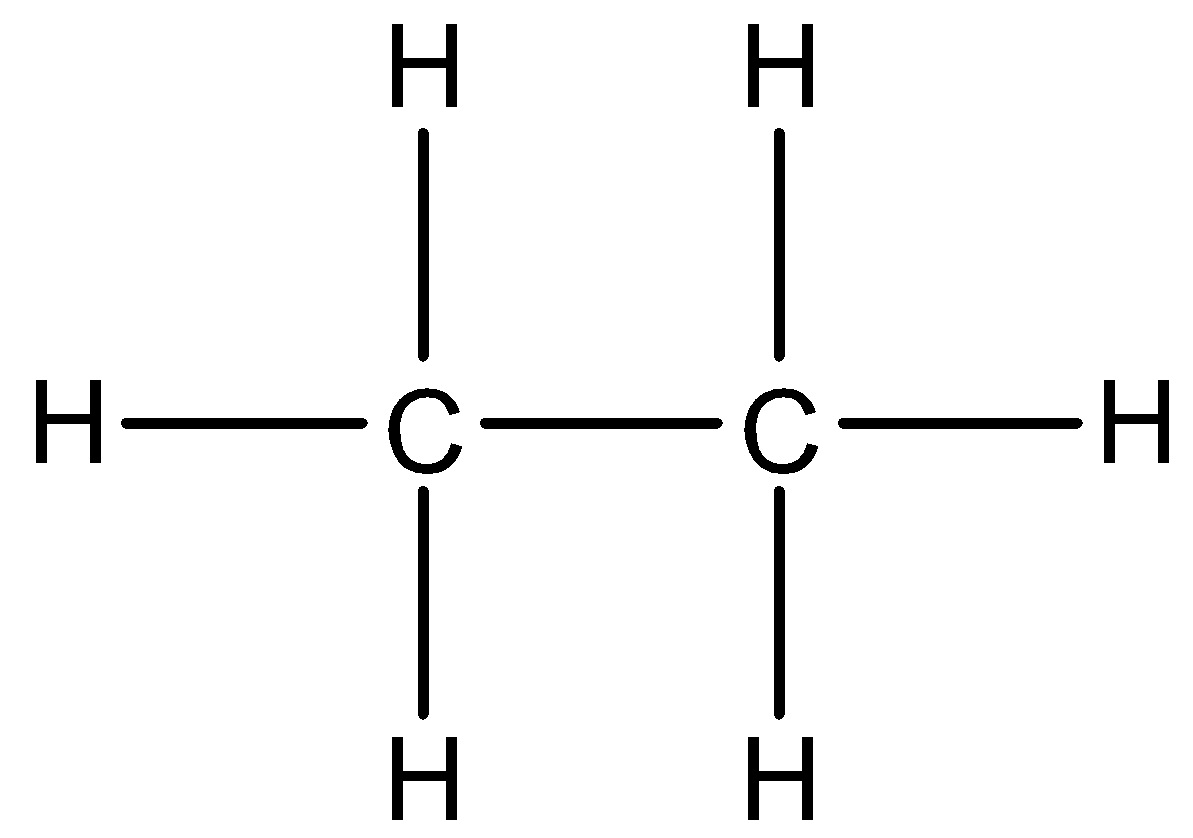
Now, counting the covalent bonds present here, we can say that there are 7 covalent bonds present.
So, the correct answer is “Option B”.
Note: Do not get confused between ionic and covalent bonds. Electrons are shared by both the atoms involved in a covalent bond. This is attributed to the negligible difference in electronegativity. Ionic bonds are where electrons are donated or received usually based on the large difference in electronegativity. Compounds involving carbon usually form covalent bonds and compounds involving metals usually form ionic bonds like salts.
Be careful while counting the bonds as we tend to ignore the C−C bond that is present due to the large number of C−H bonds.
