Question
Question: Etard's reaction involves the preparation of Benzaldehyde from: (A) Toluene (B) Ethyl benzene ...
Etard's reaction involves the preparation of Benzaldehyde from:
(A) Toluene
(B) Ethyl benzene
(C) Benzoyl chloride
(D) Sodium benzoate
Solution
Etard’s reaction involves oxidation of a methyl group attached to an aromatic system with Chromyl chloride reagent. This reaction yields corresponding aldehyde as a main product.
Complete step by step answer:
Let’s see the basics about the Etard reaction.
- Etard's reaction is a chemical reaction that involves the direct oxidation of an aromatic methyl group to an aldehyde using chromyl chloride.
- Chromyl chloride is used as an oxidising agent in this reaction. Chromium metal is in +6 oxidation state in Chromyl chloride, so it can oxidise other compounds.
- This reaction is important in synthetic organic chemistry because it oxidises methyl group to –CHO while other oxidising agents directly oxidise it to –COOH group only. Such as KMnO4,K2Cr2O7 oxidises methyl group directly to the corresponding carboxylic acid.
- The mechanism of this reaction involves a reaction followed by sigmatropic rearrangement.
For example, Toluene can be oxidized to benzaldehyde by Etard reaction.
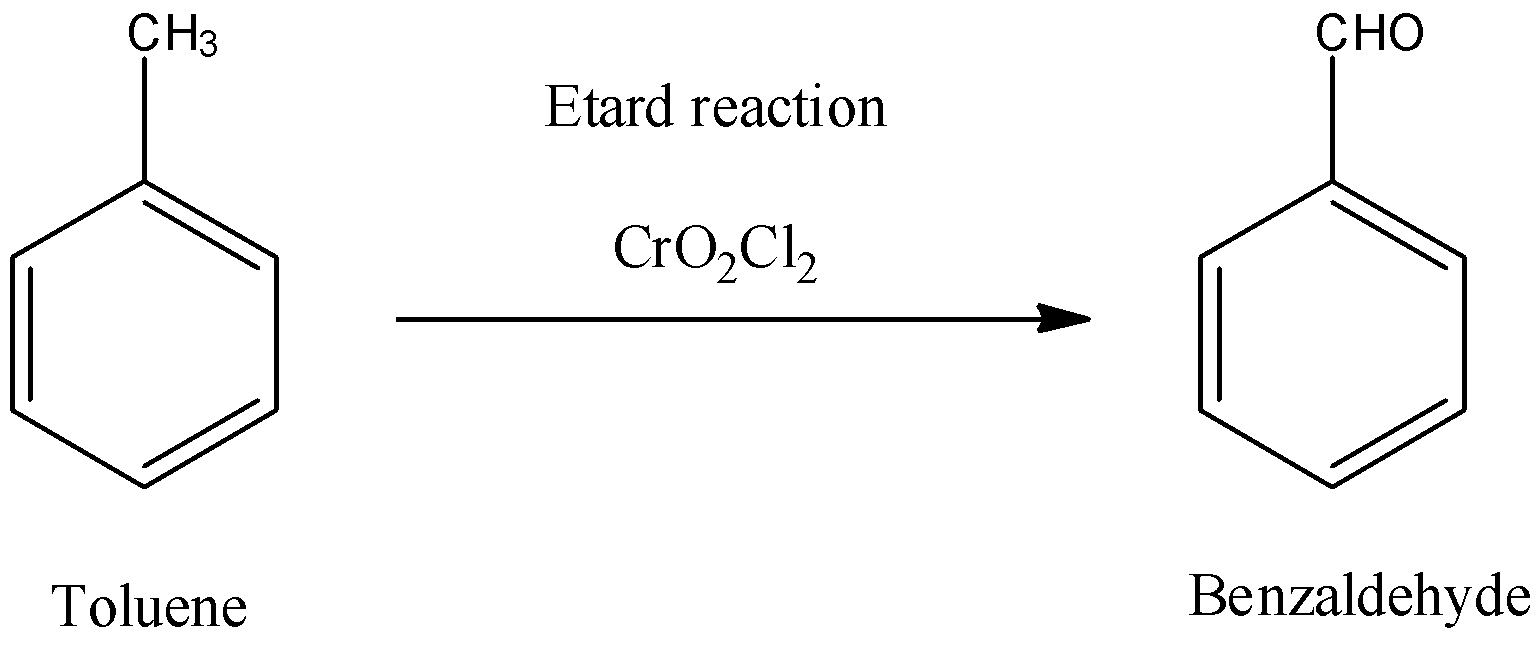
-Here, we can see that the methyl group of toluene is an aromatic methyl group and so it can be oxidised by Chromyl chloride to corresponding aldehydes. If a methyl group is attached to any aromatic heterocyclic ring, then also this reaction is possible. So, when toluene will react with Chromyl chloride, it will give benzaldehyde as a main product.
So, the correct answer is “Option C”.
Note: Remember that if an ethyl group or any other alkyl chain other than methyl is attached to the aromatic ring, then it cannot give aldehyde as a product. Instead, they will give ketone products with ketone group on the carbon which is directly attached with an aromatic ring.
